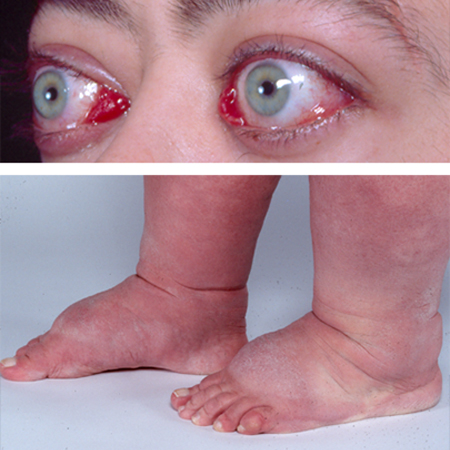
A orbitopatia de Graves ocorre em cerca de 25% dos casos e é geralmente leve.[1]Tanda ML, Piantanida E, Liparulo L, et al. Prevalence and natural history of Graves' orbitopathy in a large series of patients with newly diagnosed graves' hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013 Apr;98(4):1443-9.
https://academic.oup.com/jcem/article/98/4/1443/2536784
http://www.ncbi.nlm.nih.gov/pubmed/23408569?tool=bestpractice.com
É importante avaliar e classificar a atividade e a gravidade da orbitopatia para ajudar a orientar o tratamento.[58]Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. 2021 Aug 27;185(4):G43-G67.
https://www.doi.org/10.1530/EJE-21-0479
http://www.ncbi.nlm.nih.gov/pubmed/34297684?tool=bestpractice.com
[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
Foram publicadas diretrizes que fornecem recomendações sobre a avaliação e o tratamento iniciais.[58]Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. 2021 Aug 27;185(4):G43-G67.
https://www.doi.org/10.1530/EJE-21-0479
http://www.ncbi.nlm.nih.gov/pubmed/34297684?tool=bestpractice.com
[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
[156]Perros P, Dayan CM, Dickinson AJ, et al. Management of patients with Graves' orbitopathy: initial assessment, management outside specialised centres and referral pathways. Clin Med (Lond). 2015 Apr;15(2):173-8.
https://www.rcpjournals.org/content/clinmedicine/15/2/173
http://www.ncbi.nlm.nih.gov/pubmed/25824071?tool=bestpractice.com
Nas crianças a orbitopatia é geralmente leve e, muitas vezes, regride uma vez que se restaure o estado eutireoidiano. Uma estratégia de "esperar para ver" pode ser apropriada.
Os fatores de risco devem ser abordados para prevenir a progressão da orbitopatia de Graves, incluindo a garantia de um controle adequado da disfunção tireoidiana e o suporte aos pacientes que fumam para abandonarem o hábito de fumar.[58]Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. 2021 Aug 27;185(4):G43-G67.
https://www.doi.org/10.1530/EJE-21-0479
http://www.ncbi.nlm.nih.gov/pubmed/34297684?tool=bestpractice.com
A orbitopatia levemente ativa é tratada com colírios e pomadas lubrificantes, ou óculos de sol para sintomas superficiais, ou prismas para diplopia.[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
Alguns pacientes com orbitopatia de Graves leve de curta duração podem se beneficiar de um ciclo de 6 meses de suplementos de selênio.[157]Marcocci C, Kahaly GJ, Krassas GE, et al. Selenium and the course of mild Graves' orbitopathy. N Engl J Med. 2011 May 19;364(20):1920-31.
http://www.nejm.org/doi/full/10.1056/NEJMoa1012985#t=article
http://www.ncbi.nlm.nih.gov/pubmed/21591944?tool=bestpractice.com
[158]Bednarczuk T, Schomburg L. Challenges and perspectives of selenium supplementation in Graves' disease and orbitopathy. Hormones (Athens). 2020 Mar;19(1):31-9.
https://www.doi.org/10.1007/s42000-019-00133-5
http://www.ncbi.nlm.nih.gov/pubmed/31721133?tool=bestpractice.com
Os pacientes com orbitopatia ativa moderada a grave devem ser tratados por uma equipe multidisciplinar especializada, composta por oftalmologistas e endocrinologistas; o tratamento de primeira linha é a terapia com pulsoterapia de corticosteroides intravenosos (por exemplo, metilprednisolona).[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
[159]Zang S, Ponto KA, Kahaly GJ. Clinical review: intravenous glucocorticoids for Graves' orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011 Feb;96(2):320-32.
http://www.ncbi.nlm.nih.gov/pubmed/21239515?tool=bestpractice.com
[160]Bartalena L, Krassas GE, Wiersinga W, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves' orbitopathy. J Clin Endocrinol Metab. 2012 Dec;97(12):4454-63.
https://academic.oup.com/jcem/article/97/12/4454/2536457
http://www.ncbi.nlm.nih.gov/pubmed/23038682?tool=bestpractice.com
O uso de outros agentes, incluindo uma combinação de um corticosteroide associado a micofenolato, ou imunoterapia direcionada (por exemplo, teprotumumabe) é recomendado em alguns países.[58]Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. 2021 Aug 27;185(4):G43-G67.
https://www.doi.org/10.1530/EJE-21-0479
http://www.ncbi.nlm.nih.gov/pubmed/34297684?tool=bestpractice.com
[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
[161]American Academy of Ophthalmology. Thyroid eye disease. Jul 2024 [internet publication].
https://eyewiki.org/Thyroid_Eye_Disease
[162]Men CJ, Kossler AL, Wester ST. Updates on the understanding and management of thyroid eye disease. Ther Adv Ophthalmol. 2021 Jan-Dec;13:25158414211027760.
https://www.doi.org/10.1177/25158414211027760
http://www.ncbi.nlm.nih.gov/pubmed/34263138?tool=bestpractice.com
[163]Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017 May 4;376(18):1748-1761.
https://www.doi.org/10.1056/NEJMoa1614949
http://www.ncbi.nlm.nih.gov/pubmed/28467880?tool=bestpractice.com
[164]Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020 Jan 23;382(4):341-352.
https://www.doi.org/10.1056/NEJMoa1910434
http://www.ncbi.nlm.nih.gov/pubmed/31971679?tool=bestpractice.com
[165]Kahaly GJ, Douglas RS, Holt RJ, et al. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021 Jun;9(6):360-72.
https://www.doi.org/10.1016/S2213-8587(21)00056-5
http://www.ncbi.nlm.nih.gov/pubmed/33865501?tool=bestpractice.com
[166]Feng W, Hu Y, Zhang C, et al. Efficacy and safety of mycophenolate mofetil in the treatment of moderate to severe Graves' orbitopathy: a meta-analysis. Bioengineered. 2022 Jun;13(6):14719-29.
https://www.tandfonline.com/doi/full/10.1080/21655979.2022.2101191#d1e361
http://www.ncbi.nlm.nih.gov/pubmed/35959915?tool=bestpractice.com
Os efeitos adversos associados ao teprotumumabe incluem doença inflamatória intestinal, sintomas otológicos, incluindo perda auditiva, e hiperglicemia.[165]Kahaly GJ, Douglas RS, Holt RJ, et al. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021 Jun;9(6):360-72.
https://www.doi.org/10.1016/S2213-8587(21)00056-5
http://www.ncbi.nlm.nih.gov/pubmed/33865501?tool=bestpractice.com
[167]Kossler AL, Douglas R, Dosiou C. Teprotumumab and the evolving therapeutic landscape in thyroid eye disease. J Clin Endocrinol Metab. 2022 Aug 8;107(suppl_1):S36-46.
https://academic.oup.com/jcem/article/107/Supplement_1/S36/6658346
http://www.ncbi.nlm.nih.gov/pubmed/36346685?tool=bestpractice.com
As terapias com anticorpos monoclonais (por exemplo, rituximabe e tocilizumabe) mostraram resultados mistos e são consideradas opções de segunda ou terceira linhas.[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
[168]Salvi M, Vannucchi G, Currò N, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves' orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015 Feb;100(2):422-31.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4318899
http://www.ncbi.nlm.nih.gov/pubmed/25494967?tool=bestpractice.com
[169]Stan MN, Garrity JA, Carranza Leon BG, et al. Randomized controlled trial of rituximab in patients with Graves' orbitopathy. J Clin Endocrinol Metab. 2015 Feb;100(2):432-41.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4318907
http://www.ncbi.nlm.nih.gov/pubmed/25343233?tool=bestpractice.com
[170]Kang S, Hamed Azzam S, Minakaran N, et al. Rituximab for thyroid-associated ophthalmopathy. Cochrane Database Syst Rev. 2022 Jun 16;6(6):CD009226.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009226.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/35709102?tool=bestpractice.com
A irradiação orbital, com ou sem corticosteroides, também é usada em casos de orbitopatia ativa moderada a grave; há evidências limitadas para que se possa sugerir a radiação orbital como forma de prevenção da neuropatia óptica compressiva.[171]Chundury RV, Weber AC, Perry JD. Orbital radiation therapy in thyroid eye disease. Ophthal Plast Reconstr Surg. 2016 Mar-Apr;32(2):83-9.
http://www.ncbi.nlm.nih.gov/pubmed/26325378?tool=bestpractice.com
[172]Rajendram R, Bunce C, Lee RW, et al. Orbital radiotherapy for adult thyroid eye disease. Cochrane Database Syst Rev. 2012 Jul 11;(7):CD007114.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007114.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/22786503?tool=bestpractice.com
[173]Tanda ML, Bartalena L. Efficacy and safety of orbital radiotherapy for Graves' orbitopathy. J Clin Endocrinol Metab. 2012 Nov;97(11):3857-65.
http://www.ncbi.nlm.nih.gov/pubmed/22962421?tool=bestpractice.com
A cirurgia de reabilitação tem um papel importante na orbitopatia moderada a grave quando a doença está estável e inativa.