Investigations
1st investigations to order
FBC
Test
Should be ordered in all patients with suspected neuroblastoma as part of the initial evaluation.
Pancytopenia suggests bone marrow metastases.
Patient may need blood or platelet transfusions prior to biopsy.
Result
may reveal pancytopenia
serum electrolytes
Test
Should be ordered in all patients with suspected neuroblastoma as part of the initial evaluation.
Electrolyte abnormalities may be present due to tumour lysis syndrome, and should be treated prior to a course of chemotherapy.
Result
may be abnormal
serum creatinine/urea
Test
Should be ordered in all patients with suspected neuroblastoma as part of the initial evaluation.
May be elevated if renal vasculature is involved.
Dose modifications of contrast-based radiological studies or chemotherapy may be required.
Result
may be elevated
LFTs
Test
Should be ordered in all patients with suspected neuroblastoma as part of the initial evaluation.
Elevated LFTs may be a sign of hepatic metastases and indicate that liver function should be further evaluated.
Result
may be elevated
urine catecholamines
Test
Required for diagnosis if bone marrow is the only diagnostic tissue obtained.[32]
Catecholamine degradation products homovanillic acid (HVA) and vanillylmandelic acid (VMA) are secreted by the majority of tumours and can be detected in the patient's urine.
If elevated at diagnosis, levels should be assessed regularly during treatment and as part of end-disease surveillance.[48]
Urinary catecholamine levels are no longer recommended for the assessment of treatment response (due to influence of diet and lack of standardisation).[32][45]
Result
positive
ultrasound abdomen
Test
Should form part of the initial work-up in all patients with suspected neuroblastoma as the most common presentation is an abdominal mass.
Can confirm presence and location of mass.
If mass is detected, further imaging should include CT or MRI scan of abdomen.[32]
Result
heterogeneous mass with internal vascularity; may show calcifications or areas of necrosis
CT scan
Test
Should be ordered in all patients with suspected neuroblastoma as part of the initial evaluation.
Abdomen, chest, and pelvis should be scanned depending on location of the primary site (based on history, exam, and ultrasound). A skull/orbit scan may be required if clinically indicated.[32][Figure caption and citation for the preceding image starts]: CT scan of the abdomen showing paraspinal neuroblastomaFrom the personal collection of Dr Jason Shohet [Citation ends].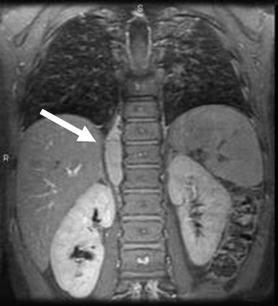 [Figure caption and citation for the preceding image starts]: CT scan showing a thoracic paraspinal neuroblastoma wrapping around the spineFrom the personal collection of Dr Jason Shohet [Citation ends].
[Figure caption and citation for the preceding image starts]: CT scan showing a thoracic paraspinal neuroblastoma wrapping around the spineFrom the personal collection of Dr Jason Shohet [Citation ends].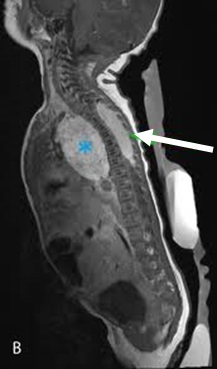
Result
heterogeneous mass; may show calcifications or areas of necrosis
MRI scan
Test
Should be ordered as an alternative to CT in all patients with suspected neuroblastoma involving the nerve roots, spinal cord or paraspinal locations as part of the initial evaluation.[32]
Abdomen, chest, pelvis, and spine should be scanned depending on location of primary site (based on history, exam, and ultrasound). A brain scan may be required if clinically indicated.[32]
Result
heterogeneous mass; may show calcifications or areas of necrosis
tumour biopsy
Test
Diagnosis is confirmed by unequivocal histology from tumour tissue by light microscopy.[32][35]
Tumour tissue is needed for diagnosis and risk stratification and is obtained by surgical resection (if indicated), incisional biopsy, or core needle biopsy.[32]
Result
characteristic pathological appearance confirms diagnosis
Investigations to consider
bilateral bone marrow aspiration and biopsy
Test
Performed in all patients to assess for bone marrow metastases.[49]
Diagnosis is confirmed by unequivocal evidence of neuroblastoma cells on bone marrow aspiration and biopsy in the setting of increased urine catecholamines.[35]
Bilateral bone marrow aspirates and trephine biopsies may be of diagnostic value when, in rare cases, marrow is the only source of tumour material (e.g., if biopsy of the primary tumour is considered to be high-risk).[32]
Result
characteristic pathological appearance confirms diagnosis
molecular genetic testing
Test
Molecular tumour profiling informs risk stratification. Techniques include fluorescence in situ hybridisation (FISH) and next-generation sequencing.
MYCN status should be assessed for all neuroblastomas. Amplification of MYCN is associated with aggressive (high-risk) disease.[32][33][34]
Presence of segmental chromosomal aberrations (e.g., 1p, 11q, 17q, 3p, 4p, 1q, and 2p) predicts poor prognosis in older children with unresectable neuroblastoma without MYCN amplification.[50]
Tumour cell DNA ploidy (number of chromosome copies) is an independent prognostic predictor in specific patient populations. Hyperdiploidy (DNA index >1) is associated with lower-risk disease and more favourable outcomes than diploidy.[51]
Result
presence or absence of specific molecular feature(s) informs patient risk stratification
serum LDH
Test
LDH may be considered.[32]
Result
may be elevated
serum vasoactive intestinal peptide (VIP)
Test
May be present at diagnosis, especially in patients with intractable diarrhoea.
Elevated in patients with tumour secretion of VIP, a paraneoplastic syndrome associated with neuroblastoma.[52]
Result
may be elevated
123-iodine-metaiodobenzylguanidine (MIBG) scintigraphy
Test
Recommended for the assessment of metastatic disease.[32] Sensitivity ranges from 67% to 100%.[53]
In approximately 10% of patients there is no uptake of 123-I-MIBG.[32][54] 18-Fluorodeoxyglucose (FDG)-PET should be obtained in the absence of 123-I-MIBG uptake or in patients with suspected mixed-avidity disease.[32]
Thyroid protection with potassium iodide should be given prior to all MIBG infusions.[47]
[Figure caption and citation for the preceding image starts]: 123-iodine-metaiodobenzylguanidine (MIBG) scintigraphy showing multifocal bony metastasesFrom the personal collection of Dr Jason Shohet [Citation ends].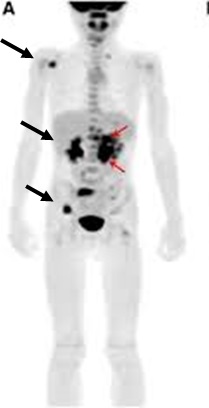
Result
identifies sites of metastases
positron emission tomography with 18-F-deoxyglucose (18F-FDG PET)
Test
Used for metastatic evaluation.
18-Fluorodeoxyglucose (FDG)-PET should be obtained in the absence of 123-I-MIBG uptake or in patients with suspected mixed-avidity disease.[32]
Result
identifies sites of metastases
Use of this content is subject to our disclaimer