The diagnosis of asthma has been the subject of debate among the major UK guideline authorities. Recommendations in this topic are based primarily on the British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) guideline, which was last updated in July 2019, but key differences from the National Institute for Health and Care Excellence (NICE) guideline are also outlined. Follow the recommended approach in your region.
The diagnosis of asthma is a clinical one.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
Diagnose asthma based on the presence of typical symptoms along with objective tests demonstrating evidence of variable airflow obstruction and/or airway inflammation.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
For information specific to diagnosing exacerbations, see Acute asthma exacerbation in adults.
Practical approach to asthma diagnosis in adults
BTS/SIGN recommend categorising the patient according to their likelihood of having asthma based on an initial structured clinical assessment aimed at identifying typical signs and symptoms and checking medical history.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Subsequent investigations and treatment trials are based on this initial probability assessment.
The flowchart below represents the step-by-step approach recommended by BTS/SIGN. Further details on each step are provided in subsequent sections.
[Figure caption and citation for the preceding image starts]: Diagnostic flowchart; reproduced from BTS/SIGN “British guideline on the management of asthma”British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. First published 2003. Revised edition published July 2019; used with permission [Citation ends].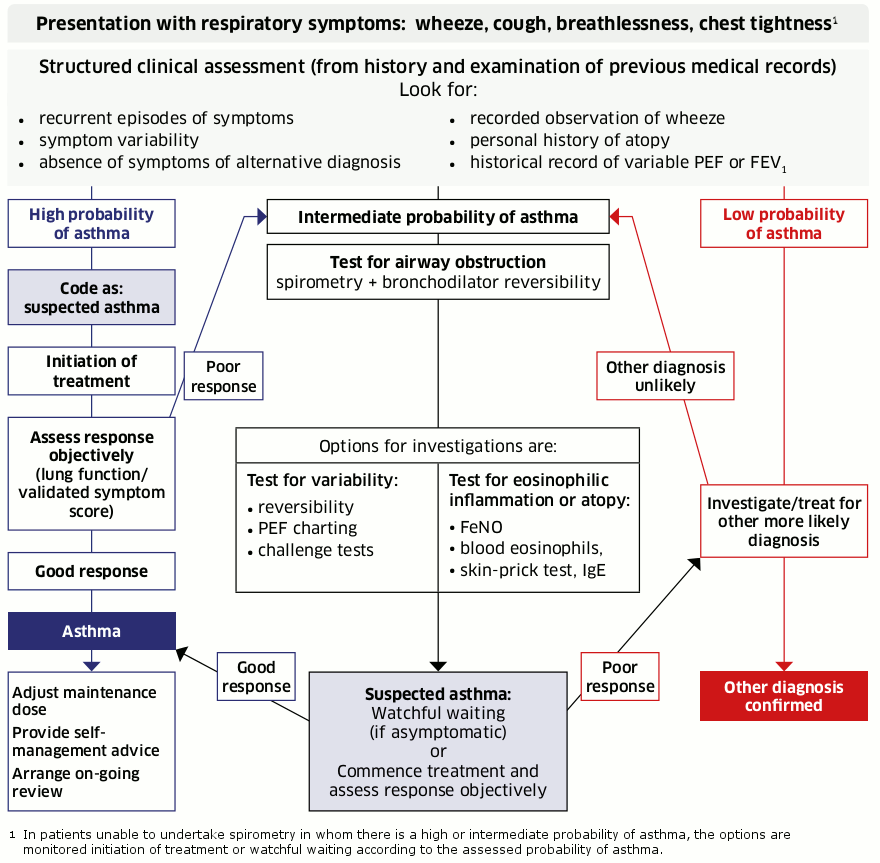
For comparison, the diagnostic path recommended by NICE is summarised in the flowchart below.[Figure caption and citation for the preceding image starts]: Objective tests for asthma in adults aged 17 and over; reproduced from NICE “Asthma: diagnosis, monitoring and chronic asthma management”National Institute for Health and Care Excellence. Algorithm C: Objective tests for asthma in adults aged 17 and over. 2017 [internet publication]; used with permission [Citation ends].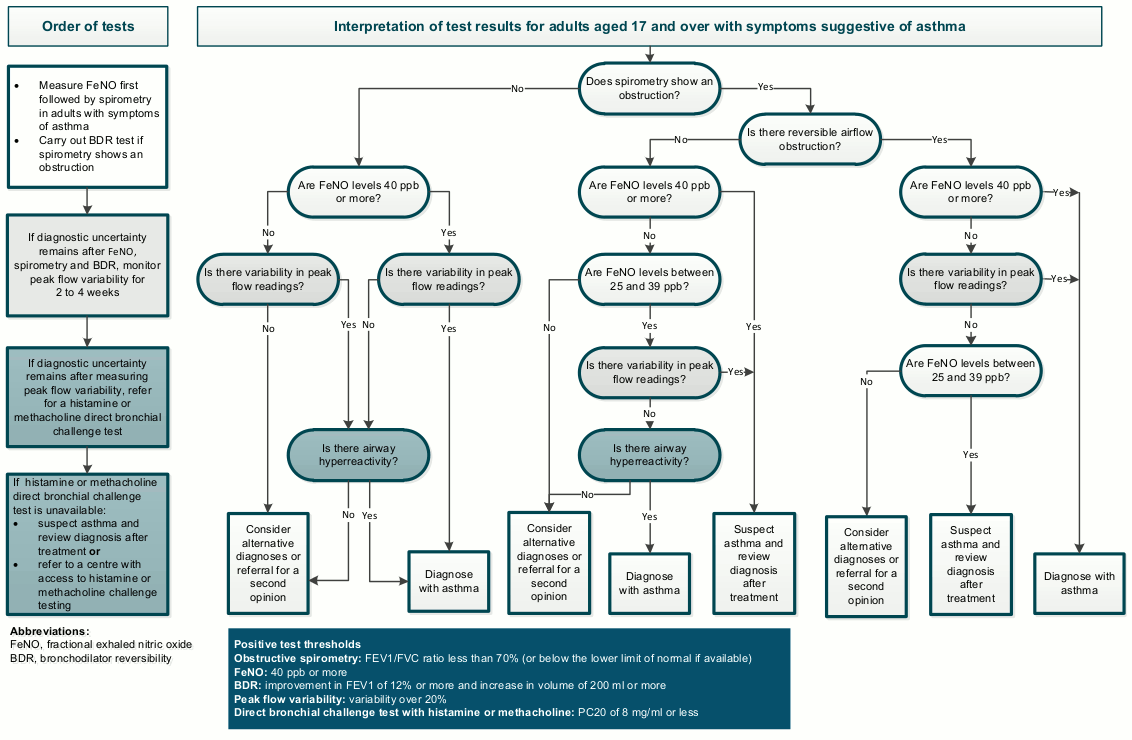
The differences from BTS/SIGN are covered in more depth in the relevant sections that follow.
Global Initiative for Asthma (GINA) guidance advises that peak expiratory flow (PEF) measurement can be used as an alternative to spirometry where spirometry services are not available. Although PEF is less reliable than spirometry, its use is preferred where diagnosis would otherwise rely on symptoms only.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
Structured clinical assessment (BTS/SIGN)
If a patient presents with symptoms suggestive of asthma, carry out a structured clinical assessment to establish whether their likelihood of having asthma is of high, intermediate, or low probability.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Base the structured clinical assessment on a combination of the history, examination, and the patient's records.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Note the disparity between BTS/SIGN and NICE recommendations; NICE also recommends a structured clinical assessment but does not refer to probabilities of asthma in its guideline. According to NICE, any individual suspected of having asthma should receive a series of objective tests before a diagnosis is made.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
BTS/SIGN recommend basing the initial probability of asthma on the following typical clinical features:[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
A history of recurrent episodes (attacks) of symptoms, ideally corroborated by differences in PEF between symptomatic and asymptomatic periods
Any two or more of the following symptoms that vary over time: wheeze; cough; breathlessness; chest tightness
Recorded observation of wheeze heard by a healthcare professional
Personal/family history of other atopic conditions (in particular, atopic eczema/dermatitis, allergic rhinitis) or family history of asthma
No symptoms/signs to suggest an alternative diagnosis.
If the patient has all of these typical clinical features, consider them to have a high probability of asthma.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Given the high probability of the patient having asthma, a trial of treatment is a pragmatic next step, particularly in symptomatic patients. See Management for more details.
An asthma diagnosis can be confirmed if the patient has a good response to treatment (both improvement in symptoms and objective improvements in lung function).
If the patient has some, but not all, of the typical features of asthma, or they have a poor response to an initial trial of treatment, assess them as having an intermediate probability of asthma.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Further investigation is needed before a diagnosis can be made, and, unless the patient is acutely unwell, before treatment is started (or continued, if the patient had a poor response to an initial trial of treatment).
Use spirometry, with bronchodilator reversibility (BDR) testing, as the next step to investigate an intermediate probability of asthma.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
See Investigations.
If the patient has none of the typical features of asthma, or presents with features more suggestive of an alternative diagnosis, the probability of asthma is low.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Bear in mind that there will be a minority of patients who will have asthma even though the presentation is atypical.
Manage low-probability patients according to the most likely differential diagnosis. See Differentials.
History and physical examination (BTS/SIGN and NICE)
Note that there are no major disparities between BTS/SIGN and NICE recommendations on history-taking and physical examination findings.
Take a careful, structured history.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Specifically check for:[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Key symptoms, which include more than one of the following: wheeze (audible on auscultation), breathlessness, chest tightness, and cough.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
Symptoms tend to occur in recurrent episodes (attacks), with periods of no (or minimal) symptoms between episodes.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
There is typically evidence of diurnal variability of symptoms (worse at night or in the early morning).[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
People with more severe asthma tend to have night-time symptoms, waking them up from sleep.
In severe exacerbations, patients are continuously short of breath and may use accessory muscles of respiration. See Acute asthma exacerbation in adults.
Triggers
Identify factors that worsen the patient's asthma: for example, episodes may be exacerbated by exposure to irritants such as tobacco smoke or fumes from chemicals such as bleach.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
Symptoms may be precipitated by infection (particularly viral), exposure to cold air, or triggered by strong reactions such as hard laughter.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
Emotions such as stress/anxiety may be associated with exacerbation of asthma symptoms, although these are more often a cause of breathing pattern disorder (dysfunctional breathing) in people with asthma.
Attacks may occur seasonally or on exposure to allergens in those with atopy.
Exercise can also trigger symptoms.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
Symptoms may be triggered by use of non-steroidal anti-inflammatory drug (NSAID) medication or beta-blockers.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
Family history of asthma or personal/family history of other atopic conditions[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
A parental history of asthma is a major risk factor for early development of asthma.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
[2]National Institutes of Health; National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3: Guidelines for the diagnosis and management of asthma. Aug 2007 [internet publication].
https://www.nhlbi.nih.gov/health-topics/guidelines-for-diagnosis-management-of-asthma
Notable other atopic conditions include atopic eczema/dermatitis, allergic rhinitis.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
The patient's historical medical records may provide valuable additional insights, including previous episodes of professionally confirmed wheeze, and past measurements of lung function or response to treatment.
Confirm wheeze on auscultation.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Distinguish wheezing (a continuous, high-pitched musical sound coming from the chest) from other respiratory noises, e.g., stridor or rattly breathing.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
With more severe asthma, the wheezes may be audible without the use of a stethoscope.
Polyphonic, high-pitched expiratory wheezes are typical of asthma.
The absence of wheeze does not exclude asthma.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Examine the nasal passages; this may reveal nasal polyposis or nasal congestion.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
Bear in mind that the examination may be normal in patients with bronchial asthma.
If the patient's chest is repeatedly normal on examination when they are symptomatic, this reduces the likelihood of asthma.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Investigations: overarching principles (BTS/SIGN versus NICE)
The key area where BTS/SIGN recommendations differ from NICE recommendations relates to the role of investigations in the asthma diagnostic pathway. Although BTS/SIGN recognise that objective tests influence the probability of asthma, they recommend that tests alone are not used to confirm the diagnosis and that in some cases a diagnosis can be confirmed without spirometry or fractional exhaled nitric oxide (FeNO) testing.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
See Diagnostic flowchart above. By contrast, NICE recommends a FeNO test followed by spirometry for any adult in whom a diagnosis of asthma is being considered based on a typical symptom presentation.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
The BTS/SIGN guideline highlights the value of comparing the results of diagnostic tests undertaken while the patient is asymptomatic with those undertaken when the patient is symptomatic. Use this to detect variation over time.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Both BTS/SIGN and NICE guidelines agree that there is no single symptom, sign, or test that can categorically determine asthma in adults.
However, NICE does not include a specific recommendation about comparing results when the patient is asymptomatic with those when a patient is symptomatic to detect variation in symptoms over time, apart from recommending PEF charting in some patients.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Performing objective tests at a time when the patient is asymptomatic may lead to false negative results. A one-off measurement of normal lung function does not exclude asthma (more than half of people with asthma may have normal spirometry).[57]Schneider A, Gindner L, Tilemann L, et al. Diagnostic accuracy of spirometry in primary care. BMC Pulm Med. 2009 Jul 10;9:31.
https://bmcpulmmed.biomedcentral.com/articles/10.1186/1471-2466-9-31
http://www.ncbi.nlm.nih.gov/pubmed/19591673?tool=bestpractice.com
[58]Ringsberg KC, Bjärneman P, Larsson R, et al. Diagnosis of asthma in primary health care: a pilot study. J Allergy (Cairo). 2014;2014:898965.
https://www.hindawi.com/journals/ja/2014/898965
http://www.ncbi.nlm.nih.gov/pubmed/24817894?tool=bestpractice.com
[59]Melbye H, Drivenes E, Dalbak LG, et al. Asthma, chronic obstructive pulmonary disease, or both? Diagnostic labeling and spirometry in primary care patients aged 40 years or more. Int J Chron Obstruct Pulmon Dis. 2011;6:597-603.
https://www.dovepress.com/asthma-chronic-obstructive-pulmonary-disease-or-both-diagnostic-labeli-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/22135492?tool=bestpractice.com
For patients with suspected asthma presenting for the first time, it is good practice to always request standard tests in the initial work-up to exclude other pathologies, including:
Spirometry and bronchodilator reversibility (BTS/SIGN and NICE)
Spirometry is the investigation of choice for identification of airflow obstruction and reversibility.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
It is usual clinical practice in the UK to arrange spirometry for every patient presenting with suspected asthma.
This is in line with NICE guidelines, which specify that you should not use symptoms alone to diagnose asthma, and that you should always use an objective test if you suspect asthma following a structured clinical assessment.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
NICE recommends FeNO followed by spirometry as essential tests for any adult with asthma symptoms.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
BDR testing is recommended by NICE if spirometry shows obstruction. NICE advises that PEF monitoring over 2 to 4 weeks may be needed if there is diagnostic uncertainty after FeNO and spirometry.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Bear in mind, however, that BTS/SIGN recommend that people who clearly have a high probability of asthma (coded as 'suspected asthma') may start an objectively monitored initiation of treatment (e.g., with PEF, validated questionnaires, and/or spirometry), and subsequently receive a confirmed diagnosis of asthma without necessarily performing spirometry or FeNO.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
BTS/SIGN guidance does recommend spirometry for any patient assessed as having an intermediate probability of asthma, with BDR testing if airway obstruction is confirmed.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Check your local guidance on the indications for spirometry in patients with suspected asthma.
The forced expiratory volume at 1 second (FEV₁) and forced vital capacity (FVC) can be used to demonstrate airflow limitation.
Use the lower limit of normal (LLN) for the FEV₁/FVC ratio rather than a fixed FEV₁/FVC ratio of 0.7; LLN is a functionality that is increasingly available through software built into spirometers.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Use of a fixed ratio can lead to overdiagnosis of obstruction in adults owing to variability of the ratio with age.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
In adults over 40 years of age, levels below 70% may be normal and use of a 70% threshold will overestimate obstruction.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
NICE also recommends using the LLN for diagnosis if available; otherwise, NICE recommends using a fixed FEV₁/FVC ratio of 70%.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Use a BDR test to demonstrate reversibility of airflow obstruction in response to short-acting bronchodilators.
BTS/SIGN and NICE agree that in adults with obstructive spirometry, an improvement in FEV₁ of 12% or more in response to beta agonists (or to a treatment trial with corticosteroids), together with an increase in volume of 200 mL or more, is regarded as a positive reversibility test.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
BTS/SIGN further recommend that an improvement of greater than 400 mL in FEV₁ strongly suggests underlying asthma.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Bear in mind that normal spirometry in an asymptomatic patient does not rule out a diagnosis of asthma.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
The false negative rate of spirometry as a diagnostic test for asthma is at least 50%.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
[57]Schneider A, Gindner L, Tilemann L, et al. Diagnostic accuracy of spirometry in primary care. BMC Pulm Med. 2009 Jul 10;9:31.
https://bmcpulmmed.biomedcentral.com/articles/10.1186/1471-2466-9-31
http://www.ncbi.nlm.nih.gov/pubmed/19591673?tool=bestpractice.com
Variability in lung function (BTS/SIGN and NICE)
Confirmation of an asthma diagnosis relies on demonstration of airflow variability over short periods of time.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Record PEF as the best of three forced expiratory blows from total lung capacity with a maximum pause of 2 seconds before blowing.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
BTS/SIGN recommend using PEF to estimate variability of airflow from multiple measurements made over 2 to 4 weeks.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Increased variability may be evident from twice-daily readings.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Bear in mind that although more frequent readings will result in a better estimate, they require improved patient adherence. If available, electronic meters and diaries with time and date stamps can improve adherence and accuracy if recording PEF in paper diaries.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
PEF variability is usually calculated as the difference between the highest and lowest PEF expressed as a percentage of the average PEF.[61]Higgins BG, Britton JR, Chinn S, et al. The distribution of peak expiratory flow variability in a population sample. Am Rev Respir Dis. 1989 Nov;140(5):1368-72.
https://www.atsjournals.org/doi/10.1164/ajrccm/140.5.1368
http://www.ncbi.nlm.nih.gov/pubmed/2817599?tool=bestpractice.com
[62]Quackenboss JJ, Lebowitz MD, Krzyzanowski M. The normal range of diurnal changes in peak expiratory flow rates. Relationship to symptoms and respiratory disease. Am Rev Respir Dis. 1991 Feb;143(2):323-30.
https://www.atsjournals.org/doi/10.1164/ajrccm/143.2.323
http://www.ncbi.nlm.nih.gov/pubmed/1990947?tool=bestpractice.com
[63]Higgins BG, Britton JR, Chinn S, et al. Comparison of bronchial reactivity and peak expiratory flow variability measurements for epidemiologic studies. Am Rev Respir Dis. 1992 Mar;145(3):588-93.
https://www.atsjournals.org/doi/10.1164/ajrccm/145.3.588
http://www.ncbi.nlm.nih.gov/pubmed/1546839?tool=bestpractice.com
However, one study showed that three or more days a week with significant variability was more sensitive and specific than calculating mean differences.[64]Thiadens HA, De Bock GH, Dekker FW, et al. Value of measuring diurnal peak flow variability in the recognition of asthma: a study in general practice. Eur Respir J. 1998 Oct;12(4):842-7.
http://www.ncbi.nlm.nih.gov/pubmed/9817156?tool=bestpractice.com
Ideally, confirm variability by comparing a PEF recorded when the patient is symptomatic (e.g., during the assessment of an asthma attack) with a PEF when asymptomatic (e.g., after recovery from an asthma attack).[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Regard a value of more than 20% variability as a positive test.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
The upper limit of the normal range for variability is around 20% using four or more PEF readings per day.[61]Higgins BG, Britton JR, Chinn S, et al. The distribution of peak expiratory flow variability in a population sample. Am Rev Respir Dis. 1989 Nov;140(5):1368-72.
https://www.atsjournals.org/doi/10.1164/ajrccm/140.5.1368
http://www.ncbi.nlm.nih.gov/pubmed/2817599?tool=bestpractice.com
[62]Quackenboss JJ, Lebowitz MD, Krzyzanowski M. The normal range of diurnal changes in peak expiratory flow rates. Relationship to symptoms and respiratory disease. Am Rev Respir Dis. 1991 Feb;143(2):323-30.
https://www.atsjournals.org/doi/10.1164/ajrccm/143.2.323
http://www.ncbi.nlm.nih.gov/pubmed/1990947?tool=bestpractice.com
NICE recommends PEF charting over 2 to 4 weeks in the following scenarios:[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
PEF diaries also have an important role in the ongoing monitoring of asthma control. See Monitoring for more details.
Fractional exhaled nitric oxide (BTS/SIGN versus NICE)
Fractional exhaled nitric oxide (FeNO) measurement can be used to find evidence of eosinophilic inflammation.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
FeNO testing is not widely available in primary care in the UK (although arrangements to change this are underway).
A positive test provides supportive, but not conclusive, evidence for a diagnosis of asthma. A negative test does not exclude the diagnosis.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
In corticosteroid-naive adults, a FeNO level of 40 ppb or more is regarded as positive.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Data from adult patients in secondary care suggest that approximately one in five people with a positive FeNO test will not have asthma (false positives), and conversely one in five people with a negative FeNO test will have asthma (false negatives).[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Bear in mind that FeNO levels are:[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[68]American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005 Apr 15;171(8):912-30.
https://www.atsjournals.org/doi/10.1164/rccm.200406-710ST
http://www.ncbi.nlm.nih.gov/pubmed/15817806?tool=bestpractice.com
[69]Alving K, Malinovschi A. Basic aspects of exhaled nitric oxide. Exhaled biomarkers. Eur Respir Monogr. 2010;49:1-31.
https://www.ers-education.org/lr/show-details/?idP=86826
[70]Bjermer L, Alving K, Diamant Z, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med. 2014 Jun;108(6):830-41.
https://www.resmedjournal.com/article/S0954-6111(14)00062-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/24636813?tool=bestpractice.com
Increased in patients with allergic rhinitis exposed to allergen, even without any respiratory symptoms
Increased by rhinovirus infection in healthy individuals, but this effect is inconsistent in people with asthma
Higher in men and tall people
Lower in children
Reduced (acutely and cumulatively) in people who smoke cigarettes[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Reduced by inhaled or oral corticosteroids[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Increased by consumption of dietary nitrates.
BTS/SIGN and NICE guidelines differ in their recommendations on the role of FeNO testing in diagnosing asthma.
BTS/SIGN recommend FeNO testing as an option, if available, for adults with an intermediary probability of asthma and normal spirometry results. In this scenario, a positive FeNO test increases the probability of asthma.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
By contrast, NICE recommends FeNO as the first-line investigation (along with spirometry) for any adult in whom a diagnosis of asthma is being considered.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Recommendations from international guidelines differ:
The American Thoracic Society (ATS) recommends FeNO as a supportive test for asthma diagnosis, stating that it should be performed if equipment is available.[71]Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011 Sep 1;184(5):602-15.
https://www.atsjournals.org/doi/full/10.1164/rccm.9120-11ST#.VBhYRvldU9I
http://www.ncbi.nlm.nih.gov/pubmed/21885636?tool=bestpractice.com
The European Respiratory Society (ERS) agrees that FeNO can be used in addition to standard tests to aid diagnosis of asthma.[72]Menzies-Gow A, Mansur AH, Brightling CE. Clinical utility of fractional exhaled nitric oxide in severe asthma management. Eur Respir J. 2020 Mar;55(3):1901633.
https://www.doi.org/10.1183/13993003.01633-2019
http://www.ncbi.nlm.nih.gov/pubmed/31949116?tool=bestpractice.com
The Global Initiative for Asthma (GINA), however, does not recommend FeNO as a test for ruling in or ruling out a diagnosis of asthma. GINA cites the overlap between FeNO levels among people with and without asthma as the basis for this recommendation. GINA asserts that the main role of FeNO is to guide treatment decisions in patients with severe asthma.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
FeNO, when used in combination with sputum eosinophilia, has a high sensitivity and specificity.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[71]Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011 Sep 1;184(5):602-15.
https://www.atsjournals.org/doi/full/10.1164/rccm.9120-11ST#.VBhYRvldU9I
http://www.ncbi.nlm.nih.gov/pubmed/21885636?tool=bestpractice.com
[73]Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011 Sep 10;378(9795):983-90.
http://www.ncbi.nlm.nih.gov/pubmed/21907861?tool=bestpractice.com
[74]Honkoop PJ, Loijmans RJ, Termeer EH, et al; Asthma Control Cost-Utility Randomized Trial Evaluation (ACCURATE) Study Group. Symptom-and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015 Mar;135(3):682-8.
http://www.ncbi.nlm.nih.gov/pubmed/25174865?tool=bestpractice.com
However, sputum eosinophilia is also not a standard test in primary care in the UK at present. Two Cochrane systematic reviews looking at tailoring asthma therapy to either sputum eosinophils or FeNO levels showed fewer exacerbations in each group, but no significant difference in all other outcomes, including quality of life, FeNO levels, or inhaled corticosteroid dose.[75]Petsky HL, Kew KM, Turner C, et al. Exhaled nitric oxide levels to guide treatment for adults with asthma. Cochrane Database Syst Rev. 2016 Sep 1;(9):CD011440.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011440.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/27580628?tool=bestpractice.com
[76]Petsky HL, Li A, Chang AB. Tailored interventions based on sputum eosinophils versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2017 Aug 24;(8):CD005603.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD005603.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/28837221?tool=bestpractice.com
Airway hyper-reactivity measures (BTS/SIGN versus NICE)
Direct bronchial challenge tests are the most widely used method of measuring airway responsiveness. They measure response in terms of change in FEV₁ a set time after the patient has inhaled increasing concentrations of bronchoconstrictor (histamine or methacholine).[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
A provocative concentration (PC20) of bronchoconstrictor of 8 mg/mL or less is regarded as positive.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
BTS/SIGN recommend to consider referring the patient for direct challenge tests if there is no evidence of airflow obstruction on initial assessment and other objective tests are inconclusive but asthma remains an intermediate possibility.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
The guideline also highlights the potential value of indirect challenge tests such as exercise and inhaled mannitol in assessing variability in lung function.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
A positive response, e.g., a fall in FEV₁ of greater than 15%, is a specific marker of asthma. Indirect challenges are less sensitive than direct challenges, particularly in patients tested while on treatment.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[77]Joos GF, O'Connor B, Anderson SD, et al. Indirect airway challenges. Eur Respir J. 2003 Jun;21(6):1050-68.
https://erj.ersjournals.com/content/21/6/1050.long
http://www.ncbi.nlm.nih.gov/pubmed/12797503?tool=bestpractice.com
[78]Anderton RC, Cuff MT, Frith PA, et al. Bronchial responsiveness to inhaled histamine and exercise. J Allergy Clin Immunol. 1979 May;63(5):315-20.
https://www.jacionline.org/article/0091-6749(79)90125-8/pdf
http://www.ncbi.nlm.nih.gov/pubmed/429710?tool=bestpractice.com
NICE recommends a direct bronchial challenge test if there is diagnostic uncertainty after a normal spirometry and either a:[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
NICE recommends to consider a direct challenge test with histamine or methacholine in people with all of the following:[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Obstructive spirometry without BDR
A FeNO level between 25 ppb and 39 ppb
No variability in PEF readings (less than 20% variability over 2-4 weeks).
NICE does not recommend indirect challenges with exercise as diagnostic tests.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Atopic status (BTS/SIGN and NICE)
Although not recommended as routine diagnostic tests, BTS/SIGN advise using a previous record of skin-prick tests, blood eosinophilia of 4% or more, or a raised allergen-specific immunoglobulin E (IgE) to corroborate a history of atopic status.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
NICE also does not recommend these as routine diagnostic tests but suggests using skin-prick tests to aeroallergens or specific IgE tests to identify triggers after a formal diagnosis of asthma has been made.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
In clinical practice, specific allergen sensitivity screening should be considered to confirm allergy in patients with a history suggestive of an allergic trigger.
If you suspect an occupational cause, see Occupational asthma.





