For information specific to management of exacerbations, see Acute asthma exacerbation in adults.
The main goal of asthma treatment is to achieve maximum control of symptoms with the fewest medications. The British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) define complete control of asthma as:[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
No daytime symptoms
No night-time waking due to asthma
No need for rescue medication
No asthma attacks/exacerbations
No limitations on activity, including exercise
Normal lung function (in practical terms forced expiratory volume at 1 second [FEV₁] and/or peak expiratory flow [PEF] more than 80% predicted or best)
Minimal adverse effects from medication
The National Institute for Health and Care Excellence (NICE) defines uncontrolled asthma as any one of:[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Three or more days a week with symptoms
Three or more days a week with required use of a short-acting beta agonist (SABA) for symptomatic relief
One or more nights a week with waking due to asthma
In practice, complete control of asthma may require a regimen that is inconvenient for the patient and may lead to adverse effects. The goal of complete control should therefore be balanced with the patient's lifestyle and their tolerance for adverse effects.
Once control of symptoms is achieved, attempt to reduce the doses of medications while maintaining optimal control and minimising any adverse effects.
Education and environmental control
Ensure all patients at all steps of therapy have access to a self-management programme, which should include a written personalised asthma action plan and education.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
[85]Gatheral TL, Rushton A, Evans DJ, et al. Personalised asthma action plans for adults with asthma. Cochrane Database Syst Rev. 2017 Apr 10;(4):CD011859.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011859.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/28394084?tool=bestpractice.com
[  ]
What are the effects of personalized asthma action plans in adults with mild, moderate, or severe asthma?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1796/fullShow me the answer This should be supported by regular professional review.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Advise the patient to take environmental control measures (e.g., reduce exposure to indoor and outdoor air pollution, tobacco smoke, and occupational and domestic allergens).[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
If you suspect an occupational cause, see Occupational asthma.
]
What are the effects of personalized asthma action plans in adults with mild, moderate, or severe asthma?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1796/fullShow me the answer This should be supported by regular professional review.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Advise the patient to take environmental control measures (e.g., reduce exposure to indoor and outdoor air pollution, tobacco smoke, and occupational and domestic allergens).[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
If you suspect an occupational cause, see Occupational asthma.
Consider breathing exercise programmes as part of an integrated approach to management, alongside pharmacological treatment, to improve the patient's quality of life and reduce symptoms.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Approaches used in practice include the Papworth method and the Buteyko method.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
These techniques involve instruction by a trained therapist in exercises to reduce respiratory rate and minute volume, and to promote nasal, diaphragmatic breathing.
Breathing exercises can lead to modest improvements in asthma symptoms and quality of life, and reduce bronchodilator requirement in adults with asthma, but have little effect on lung function or airway inflammation.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[86]Bruton A, Lee A, Yardley L, et al. Physiotherapy breathing retraining for asthma: a randomised controlled trial. Lancet Respir Med. 2018 Jan;6(1):19-28.
https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(17)30474-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29248433?tool=bestpractice.com
[87]O’Connor E, Patnode CD, Burda BU, et al. Breathing exercises and/or retraining techniques in the treatment of asthma: comparative effectiveness [internet]. (Comparative Effectiveness Reviews, No. 71.) Rockville, MD: Agency for Healthcare Research and Quality (US); Sep 2012.
https://www.ncbi.nlm.nih.gov/books/NBK109355
http://www.ncbi.nlm.nih.gov/pubmed/23101047?tool=bestpractice.com
[88]Santino TA, Chaves GS, Freitas DA, et al. Breathing exercises for adults with asthma. Cochrane Database Syst Rev. 2020 Mar 25;(3):CD001277.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001277.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/32212422?tool=bestpractice.com
[89]Prem V, Sahoo RC, Adhikari P. Comparison of the effects of Buteyko and pranayama breathing techniques on quality of life in patients with asthma - a randomized controlled trial. Clin Rehabil. 2013 Feb;27(2):133-41.
http://www.ncbi.nlm.nih.gov/pubmed/22837543?tool=bestpractice.com
More studies are needed.[88]Santino TA, Chaves GS, Freitas DA, et al. Breathing exercises for adults with asthma. Cochrane Database Syst Rev. 2020 Mar 25;(3):CD001277.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001277.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/32212422?tool=bestpractice.com
[  ]
What are the effects of breathing exercises for adults with asthma?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3095/fullShow me the answer
]
What are the effects of breathing exercises for adults with asthma?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3095/fullShow me the answer
Exercise-induced bronchoconstriction
Always review the patient's regular treatment and check their inhaler technique and adherence if they are experiencing exercise-induced symptoms; breakthrough exercise-induced bronchoconstriction may indicate poorly controlled asthma, potentially requiring stepping up of treatment (see Stepwise therapy for long-term management).[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[  ]
What are the effects of interventions to improve inhaler technique for adults with asthma?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2623/fullShow me the answer
]
What are the effects of interventions to improve inhaler technique for adults with asthma?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2623/fullShow me the answer
If exercise induces symptoms (e.g., shortness of breath, wheezing) in patients whose asthma is otherwise well controlled with an inhaled corticosteroid (ICS) or other stepwise therapy (see below), advise the patient to use an inhaled SABA immediately before exercise.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
In addition, consider adding one of the following to their usual medication:[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Leukotriene receptor antagonist (LTRA)
Long-acting beta agonist (LABA)
Sodium cromoglicate or nedocromil[90]Kelly K, Spooner CH, Rowe BH. Nedocromil sodium vs. sodium cromoglycate for preventing exercise-induced bronchoconstriction in asthmatics. Cochrane Database Syst Rev. 2000 Jul 24;(4):CD002731.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002731/full
http://www.ncbi.nlm.nih.gov/pubmed/11034750?tool=bestpractice.com
Theophylline[91]Nassif EG, Weinberger M, Thompson R, et al. The value of maintenance theophylline in steroid-dependent asthma. N Engl J Med. 1981 Jan 8;304(2):71-5.
http://www.ncbi.nlm.nih.gov/pubmed/7003383?tool=bestpractice.com
The Medicines and Healthcare products Regulatory Agency (MHRA) warns of serious behaviour- and mood-related adverse effects with montelukast (an LTRA), and advises that healthcare professionals:[92]Medicines and Healthcare products Regulatory Agency. Montelukast: reminder of the risk of neuropsychiatric reactions. Apr 2024 [internet publication].
https://www.gov.uk/drug-safety-update/montelukast-reminder-of-the-risk-of-neuropsychiatric-reactions
Be alert for neuropsychiatric reactions in patients taking montelukast, including but not limited to sleep disturbances, depression and agitation, disturbances of attention or memory, speech impairment (stuttering), and obsessive-compulsive symptoms.
Advise patients and their carers to read carefully the list of neuropsychiatric reactions in the patient information leaflet and seek medical advice immediately should they occur.
Evaluate carefully the risks and benefits of continuing treatment if neuropsychiatric reactions occur.
Stepwise therapy for long-term management
Guidelines recommend that asthma severity and control be viewed as a ladder in which medication can be stepped up or stepped down based on the severity of the disease and adequacy of the control.
The patient's symptoms should be assessed and recorded on at least an annual basis in a clinical review by a healthcare professional with appropriate training in asthma management. These reviews can be undertaken in primary and/or secondary care according to local availability and clinical need.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
For more information on annual reviews, see Monitoring section.
When assessing the patient's symptoms, use specific questions, such as the Royal College of Physicians' '3 Questions' (RCP3Q):[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
'In the last month, have you had difficulty sleeping because of asthma symptoms (including cough)?'[93]Clinical Effectiveness and Evaluation Unit, Pearson M, Bucknail C, eds. Measuring clinical outcome in asthma: a patient-focused approach. London: Royal College of Physicians; 1999.
'Have you had your usual asthma symptoms during the day?'[93]Clinical Effectiveness and Evaluation Unit, Pearson M, Bucknail C, eds. Measuring clinical outcome in asthma: a patient-focused approach. London: Royal College of Physicians; 1999.
'Has your asthma interfered with your usual activities (e.g., housework, work, etc.)?'[93]Clinical Effectiveness and Evaluation Unit, Pearson M, Bucknail C, eds. Measuring clinical outcome in asthma: a patient-focused approach. London: Royal College of Physicians; 1999.
Positive responses to RCP3Q should prompt further assessment with a validated questionnaire to measure symptomatic asthma control, for example the Asthma Control Questionnaire (ACQ) or Asthma Control Test (ACT).[2]National Institutes of Health; National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3: Guidelines for the diagnosis and management of asthma. Aug 2007 [internet publication].
https://www.nhlbi.nih.gov/health-topics/guidelines-for-diagnosis-management-of-asthma
[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
[94]Ahmad S, Kew KM, Normansell R. Stopping long-acting beta2-agonists (LABA) for adults with asthma well controlled by LABA and inhaled corticosteroids. Cochrane Database Syst Rev. 2015 Jun 19;(6):CD011306.
https://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011306.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/26089258?tool=bestpractice.com
[  ]
What are the effects of stepping down the dose of inhaled corticosteroids for adults with asthma?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1738/fullShow me the answer
]
What are the effects of stepping down the dose of inhaled corticosteroids for adults with asthma?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1738/fullShow me the answer
The stepwise approach is meant to assist, not replace, the clinical decision making required to meet individual patient needs.
Patients may start at any step of the ladder, according to their initial severity, and medications can be added (stepped up) if needed.
Stepping up of treatment may be needed at any time in a patient with poor symptom control and/or exacerbations despite taking asthma treatment. Before stepping up, always check the patient's inhaler technique and adherence to treatment, confirm the diagnosis of asthma, work with the patient to reduce or remove any triggers (e.g., smoking), and address comorbidities where feasible.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[95]Normansell R, Kew KM, Mathioudakis AG. Interventions to improve inhaler technique for people with asthma. Cochrane Database Syst Rev. 2017 Mar 13;(3):CD012286.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012286.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/28288272?tool=bestpractice.com
[  ]
What are the effects of interventions to improve inhaler technique for adults with asthma?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2623/fullShow me the answer In the UK, NICE recommends allowing 4 to 8 weeks to assess the patient's response before making a decision on whether a step up to the next level of treatment is needed.[96]National Institute for Health and Care Excellence. Algorithm F: pharmacological treatment of chronic asthma in adults aged 17 and over. 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80/resources/algorithm-f-pharmacological-treatment-of-chronic-asthma-in-adults-aged-17-and-over-pdf-4656176754
]
What are the effects of interventions to improve inhaler technique for adults with asthma?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2623/fullShow me the answer In the UK, NICE recommends allowing 4 to 8 weeks to assess the patient's response before making a decision on whether a step up to the next level of treatment is needed.[96]National Institute for Health and Care Excellence. Algorithm F: pharmacological treatment of chronic asthma in adults aged 17 and over. 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80/resources/algorithm-f-pharmacological-treatment-of-chronic-asthma-in-adults-aged-17-and-over-pdf-4656176754
Increasing use of a SABA (if prescribed) or use >2 days a week for symptom relief (not prevention of exercise-induced bronchoconstriction) generally indicates inadequate control and the need to step up treatment.
Regularly assess the patient's asthma control, with the aim of stepping down the ladder if their symptoms have been well controlled for at least 3 months.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Ongoing regular review of patients as treatment is decreased is important.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
When deciding which drug to decrease first and at what rate, take into account: the severity of asthma, the adverse effects of the treatment, time on current dose, the beneficial effect achieved, and the patient's preference.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Patients should be maintained at the lowest possible dose of ICS. Reduction in dose should be slow as patients deteriorate at different rates. Reductions should be considered every 3 months, decreasing the dose by approximately 25% to 50% each time.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
The management of asthma has been the subject of debate among the major UK guideline authorities, with differences in the details of their step-up pharmacological options. Recommendations in this topic are based primarily on the BTS/SIGN guideline, which was last updated in July 2019, but a summary of the NICE guideline is also provided towards the end of this section. Follow the recommended approach in your region.
[Figure caption and citation for the preceding image starts]: Summary of management in adults; reproduced from BTS/SIGN “British guideline on the management of asthma”British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. First published 2003. Revised edition published July 2019; used with permission [Citation ends].
BTS/SIGN: step 1 - initial therapy for control of asthma
SABA
Prescribe an inhaled SABA (e.g., salbutamol), as short-term therapy, to be used as needed to relieve symptoms in symptomatic patients.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
In practice, only a very limited number of patients will need occasional use of SABAs alone with no preventer therapy. Most patients will also need regular preventer therapy with ICS.
SABAs work more quickly and/or with fewer adverse effects than the alternatives (ipratropium, theophylline).[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[97]Eccles M, Rousseau N, Higgins B, et al. Evidence-based guideline on the primary care management of asthma. Fam Pract. 2001 Apr;18(2):223-9.
https://academic.oup.com/fampra/article/18/2/223/492398
http://www.ncbi.nlm.nih.gov/pubmed/11264277?tool=bestpractice.com
If the patient needs more than one short-acting bronchodilator inhaler device a month, arrange urgent assessment of their asthma and take measures to improve asthma control if this is poor.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
High SABA use is associated with a significant increase in exacerbations and asthma-related healthcare utilisation.[98]Bloom CI, Cabrera C, Arnetorp S, et al. Asthma-related health outcomes associated with short-acting β2-agonist inhaler use: an observational UK study as part of the SABINA global program. Adv Ther. 2020 Oct;37(10):4190-208.
https://link.springer.com/article/10.1007%2Fs12325-020-01444-5
http://www.ncbi.nlm.nih.gov/pubmed/32720299?tool=bestpractice.com
[99]Amin S, Soliman M, McIvor A, et al. Usage patterns of short-acting β2-agonists and inhaled corticosteroids in asthma: a targeted literature review. J Allergy Clin Immunol Pract. 2020 Sep;8(8):2556-64.
http://www.ncbi.nlm.nih.gov/pubmed/32244024?tool=bestpractice.com
Overuse of SABAs is also a risk factor for fatal asthma.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Patient populations most at risk for SABA over-reliance include older adults, smokers, and patients with lower socioeconomic status.[99]Amin S, Soliman M, McIvor A, et al. Usage patterns of short-acting β2-agonists and inhaled corticosteroids in asthma: a targeted literature review. J Allergy Clin Immunol Pract. 2020 Sep;8(8):2556-64.
http://www.ncbi.nlm.nih.gov/pubmed/32244024?tool=bestpractice.com
Low-dose ICS
Consider adding low-dose ICS to SABA if the patient has any of the following asthma-related features:[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Acute asthma attack (requiring oral corticosteroids) in the past 2 years
Using inhaled SABA three times a week or more
Symptomatic three times a week or more
Waking one night a week
Although alternatives are available (e.g., sodium cromoglicate, nedocromil, theophylline), ICSs are the most effective preventer drug for achieving overall treatment goals.[100]Scottish Intercollegiate Guidelines Network (SIGN). Pharmacological management of asthma. Evidence table 4.4a: inhaled corticosteroid vs theophylline. Jul 2019 [internet publication].
https://www.sign.ac.uk/our-guidelines/british-guideline-on-the-management-of-asthma/british-guideline-on-the-management-of-asthma-evidence-tables
[101]Scottish Intercollegiate Guidelines Network (SIGN). Pharmacological management of asthma. Evidence table 4.4c: inhaled corticosteroid vs leukotriene receptor antagonists. Jul 2019 [internet publication].
https://www.sign.ac.uk/our-guidelines/british-guideline-on-the-management-of-asthma/british-guideline-on-the-management-of-asthma-evidence-tables
[102]Adams NP, Bestall JC, Lasserson TJ, et al. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database Syst Rev. 2008 Oct 8;(4):CD003135.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003135.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/18843640?tool=bestpractice.com
[103]Calpin C, Macarthur C, Stephens D, et al. Effectiveness of prophylactic inhaled steroids in childhood asthma: a systemic review of the literature. J Allergy Clin Immunol. 1997 Oct;100(4):452-7.
http://www.ncbi.nlm.nih.gov/pubmed/9338536?tool=bestpractice.com
Adding ICS to SABA significantly reduces the risk of severe exacerbations and asthma-related death associated with overuse of SABA.[1]Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 [internet publication].
https://ginasthma.org/reports
[104]Nwaru BI, Ekström M, Hasvold P, et al. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020 Apr;55(4):1901872.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7160635
http://www.ncbi.nlm.nih.gov/pubmed/31949111?tool=bestpractice.com
[105]Stanford RH, Shah MB, D'Souza AO, et al. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. 2012 Dec;109(6):403-7.
http://www.ncbi.nlm.nih.gov/pubmed/23176877?tool=bestpractice.com
Start the patient at a reasonable starting dose of ICS appropriate to the severity of their asthma.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Titrate to the lowest dose at which effective control of asthma is maintained.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Higher doses might be needed in patients who smoke or used to smoke.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
BTS/SIGN: step 2 - initial add-on therapy
If the patient's symptoms are not adequately controlled with low-dose ICS alone, BTS/SIGN recommend to add a LABA, either as a fixed-dose regimen (with SABA as needed) or as combination maintenance and reliever therapy in a single inhaler (MART).[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
The addition of an inhaled LABA to ICS alone improves lung function and symptoms, and decreases asthma attacks.[106]Chauhan BF, Jeyaraman MM, Singh Mann A, et al. Addition of anti-leukotriene agents to inhaled corticosteroids for adults and adolescents with persistent asthma. Cochrane Database Syst Rev. 2017 Mar 16;3(3):CD010347.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010347.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/28301050?tool=bestpractice.com
Before stepping up to a new drug, always check the patient's inhaler technique and adherence to treatment, confirm the diagnosis of asthma, work with the patient to reduce or remove any triggers (e.g., smoking), and address comorbidities where feasible.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Option A: fixed-dose LABA + low-dose ICS + SABA as needed
Prescribe a fixed-dose combination ICS/LABA inhaler.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
LABAs should never be used without ICS.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
LABA monotherapy is associated with an increased risk of adverse events, life-threatening asthma, and asthma hospitalisation events.[107]Rodrigo GJ, Castro-Rodríguez JA. Safety of long-acting β agonists for the treatment of asthma: clearing the air. Thorax. 2012 Apr;67(4):342-9.
https://thorax.bmj.com/content/67/4/342.long
http://www.ncbi.nlm.nih.gov/pubmed/21515554?tool=bestpractice.com
[108]Currie GP, Small I, Douglas G. Long acting β2 agonists in adult asthma. BMJ. 2013 Aug 6;347:f4662.
http://www.ncbi.nlm.nih.gov/pubmed/23920253?tool=bestpractice.com
Experience in clinical practice shows that combination inhalers not only help patient adherence but also ensure that the LABA is not taken without the ICS.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
The patient should continue with their intermittent reliever therapy (usually an inhaled SABA; see step 1: initial therapy for control of asthma), used as needed.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Option B: MART (LABA + low-dose ICS)
Consider MART, particularly if the patient has a history of asthma attacks on a fixed-dose LABA and a low-dose ICS.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
MART allows for the rapid onset of a reliever effect with formoterol; by also including a dose of ICS, MART ensures that the dose of preventer medication increases as the need for a reliever increases.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Therefore, a comprehensive self-management plan must be provided with a MART regimen.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
MART may also lower the overall dose of ICS needed to prevent asthma attacks.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
The patient should not use intermittent reliever therapy (e.g., an inhaled SABA) alongside MART.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
One Cochrane review of serious adverse events when taking ICS with and without regular formoterol found no difference in risk of death in adults taking ICS-formoterol versus ICS alone.[109]Janjua S, Schmidt S, Ferrer M, et al. Inhaled steroids with and without regular formoterol for asthma: serious adverse events. Cochrane Database Syst Rev. 2019 Sep 25;(9):CD006924.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006924.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/31553802?tool=bestpractice.com
[  ]
For adults with asthma who are taking inhaled steroids, what serious adverse events are associated with formoterol?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2784/fullShow me the answer
]
For adults with asthma who are taking inhaled steroids, what serious adverse events are associated with formoterol?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2784/fullShow me the answer
BTS/SIGN: step 3 - additional controller therapies
If symptom control remains suboptimal after initial add-on therapy, BTS/SIGN recommend either increasing the ICS to medium dose or adding an LTRA.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Before stepping up treatment, always check the patient's inhaler technique and adherence to treatment, confirm the diagnosis of asthma, work with the patient to reduce or remove any triggers (e.g., smoking), and address comorbidities where feasible.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Option A: medium-dose ICS
If there was some improvement when a LABA was added as initial add-on therapy, but symptom control remains suboptimal, continue with the LABA and consider increasing the dose of ICS from low to medium (either as MART or a fixed-dose regimen).[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Consider stopping the LABA before increasing the ICS dose if there was no improvement when a LABA was added.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Bear in mind that in clinical practice, the LABA is very rarely stopped so proceed cautiously if considering this.
If the patient is taking a fixed-dose LABA, they should continue with their intermittent reliever therapy (usually an inhaled SABA; see step 1: initial therapy for control of asthma), used as needed. The patient should not use intermittent reliever therapy (e.g., an inhaled SABA) alongside MART.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Option B: add LTRA
If there was some improvement when a LABA was added as initial add-on therapy, but symptom control remains suboptimal, continue with the LABA and consider adding an LTRA (e.g., montelukast).[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
BTS/SIGN recommend to consider stopping the LABA before starting the LTRA if there was no improvement when a LABA was added.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
In clinical practice, the LABA is usually continued so proceed with caution if considering this.
If on a fixed-dose regimen, the patient should continue with their intermittent reliever therapy (usually an inhaled SABA; see step 1: initial therapy for control of asthma), used as needed.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
The MHRA warns of serious behaviour- and mood-related adverse effects of montelukast, and advises that healthcare professionals:[92]Medicines and Healthcare products Regulatory Agency. Montelukast: reminder of the risk of neuropsychiatric reactions. Apr 2024 [internet publication].
https://www.gov.uk/drug-safety-update/montelukast-reminder-of-the-risk-of-neuropsychiatric-reactions
Be alert for neuropsychiatric reactions in patients taking montelukast, including but not limited to sleep disturbances, depression and agitation, disturbances of attention or memory, speech impairment (stuttering), and obsessive-compulsive symptoms
Advise patients and their carers to read carefully the list of neuropsychiatric reactions in the patient information leaflet and seek medical advice immediately should they occur
Evaluate carefully the risks and benefits of continuing treatment if neuropsychiatric reactions occur.
BTS/SIGN: step 4 - specialist therapies
If the patient has severe poorly controlled asthma despite step 3 treatment, with good adherence and correct inhaler technique, BTS/SIGN recommend referring to a specialist.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
The specialist might try a number of approaches including:[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
High-dose ICS
A specialist may consider increasing the ICS to a high dose; this should only be done as part of a fixed-dose regimen, with a SABA used as a reliever therapy.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
LAMA
If the patient's asthma does not respond to ICS plus LABA, the addition of a LAMA (e.g., tiotropium) to ICS is a possible alternative a specialist might consider.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Theophylline
Oral theophylline is a bronchodilator which may improve lung function and symptoms, but is associated with adverse effects including headache, nausea, and vomiting.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Oral corticosteroids
Some patients with very severe asthma not controlled with high-dose ICS, and who have also been trialled or are still taking LABA, LTRA, LAMA, or theophylline, may require regular long-term oral corticosteroids.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
In practice, only a small number of patients will have symptoms that remain uncontrolled despite high-dose therapies. For these patients, daily oral corticosteroids should only be used at the lowest dose providing adequate control.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
All patients requiring frequent or continuous use of oral corticosteroids should be under the care of a specialist asthma service.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Biological agents
If the patient has severe persistent asthma that continues to be uncontrolled despite other step 4 therapies, a specialist in a tertiary care centre may consider biological agents as an add-on to optimised standard therapy. Optimised standard therapy is defined as a full trial of, and if tolerated, documented compliance with, high-dose ICS, LABA, LTRA, theophylline, oral corticosteroids, and smoking cessation if clinically appropriate.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Add-on biological agents a specialist may consider include:
Omalizumab: for patients with severe persistent allergic immunoglobulin E (IgE) mediated asthma who have:[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[110]Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011 Jan;139(1):28-35.
http://www.ncbi.nlm.nih.gov/pubmed/20688929?tool=bestpractice.com
[111]Bardelas J, Figliomeni M, Kianifard F, et al. A 26-week, randomized, double-blind, placebo-controlled, multicenter study to evaluate the effect of omalizumab on asthma control in patients with persistent allergic asthma. J Asthma. 2012 Mar;49(2):144-52.
http://www.ncbi.nlm.nih.gov/pubmed/22277052?tool=bestpractice.com
[112]Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011 May 3;154(9):573-82.
https://www.acpjournals.org/doi/10.7326/0003-4819-154-9-201105030-00002?articleid=746947
http://www.ncbi.nlm.nih.gov/pubmed/21536936?tool=bestpractice.com
[113]Norman G, Faria R, Paton F, et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess. 2013 Nov;17(52):1-342.
https://www.journalslibrary.nihr.ac.uk/hta/hta17520#/full-report
http://www.ncbi.nlm.nih.gov/pubmed/24267198?tool=bestpractice.com
[114]Hambly N, Nair P. Monoclonal antibodies for the treatment of refractory asthma. Curr Opin Pulm Med. 2014 Jan;20(1):87-94.
http://www.ncbi.nlm.nih.gov/pubmed/24275927?tool=bestpractice.com
[115]National Institute for Health and Care Excellence. Omalizumab for treating severe persistent allergic asthma. Apr 2013 [internet publication].
https://www.nice.org.uk/guidance/ta278
A positive skin test or in vitro reactivity to a perennial aeroallergen
Reduced lung function (FEV₁ less than 80%)
Frequent daytime symptoms or night-time awakenings
Multiple documented severe exacerbations despite daily high-dose ICS plus a LABA.
Mepolizumab: for severe refractory eosinophilic asthma if:[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[114]Hambly N, Nair P. Monoclonal antibodies for the treatment of refractory asthma. Curr Opin Pulm Med. 2014 Jan;20(1):87-94.
http://www.ncbi.nlm.nih.gov/pubmed/24275927?tool=bestpractice.com
[116]National Institute for Health and Care Excellence. Mepolizumab for treating severe eosinophilic asthma. Feb 2021 [internet publication].
https://www.nice.org.uk/guidance/ta671
The patient's blood eosinophil count has been recorded as ≥300 cells per microlitre and they had at least 4 exacerbations needing systemic corticosteroids in the previous 12 months, or the patient has had continuous oral corticosteroids of at least the equivalent of prednisolone 5 mg/day over the previous 6 months, or
The patient's blood eosinophil count has been recorded as ≥400 cells per microlitre and they have had at least 3 exacerbations needing systemic corticosteroids in the previous 12 months (so the patient is also eligible for either benralizumab or reslizumab - see below).
Reslizumab: for severe eosinophilic asthma that is inadequately controlled despite maintenance therapy with high-dose ICS plus another drug, only if:[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[114]Hambly N, Nair P. Monoclonal antibodies for the treatment of refractory asthma. Curr Opin Pulm Med. 2014 Jan;20(1):87-94.
http://www.ncbi.nlm.nih.gov/pubmed/24275927?tool=bestpractice.com
[117]National Institute for Health and Care Excellence. Reslizumab for treating severe eosinophilic asthma. Oct 2017 [internet publication].
https://www.nice.org.uk/guidance/ta479
Benralizumab: for treating severe eosinophilic asthma that remains uncontrolled despite maintenance therapy with high-dose ICS and LABA, only if:[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
[114]Hambly N, Nair P. Monoclonal antibodies for the treatment of refractory asthma. Curr Opin Pulm Med. 2014 Jan;20(1):87-94.
http://www.ncbi.nlm.nih.gov/pubmed/24275927?tool=bestpractice.com
[118]Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013 Nov;132(5):1086-96.
http://www.ncbi.nlm.nih.gov/pubmed/23866823?tool=bestpractice.com
[119]National Institute for Health and Care Excellence. Benralizumab for treating severe eosinophilic asthma. Mar 2019 [internet publication].
https://www.nice.org.uk/guidance/ta565
The patient's blood eosinophil count has been recorded as ≥300 cells per microlitre and the person has had 4 or more exacerbations needing systemic corticosteroids in the previous 12 months, or has had continuous oral corticosteroids of at least the equivalent of prednisolone 5 mg/day over the previous 6 months (i.e., the patient is eligible for mepolizumab), or
The patient's blood eosinophil count has been recorded as ≥400 cells per microlitre with 3 or more exacerbations needing systemic corticosteroids in the past 12 months (i.e., the patient is eligible for reslizumab).
Dupilumab: for patients with severe asthma with type 2 inflammation that is inadequately controlled, despite maintenance therapy with high-dose ICS and another maintenance treatment, only if:[120]Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013 Jun 27;368(26):2455-66.
https://www.nejm.org/doi/full/10.1056/NEJMoa1304048
http://www.ncbi.nlm.nih.gov/pubmed/23688323?tool=bestpractice.com
[121]National Institute for Health and Care Excellence. Dupilumab for treating severe asthma with type 2 inflammation. Dec 2021 [internet publication].
https://www.nice.org.uk/guidance/ta751
The patient has a blood eosinophil count of ≥150 cells per microlitre and fractional exhaled nitric oxide of ≥25 parts per billion, and has had at least 4 or more exacerbations in the previous 12 months.
The patient is not eligible for mepolizumab, reslizumab, or benralizumab, or has asthma that has not responded adequately to these biological therapies.
Tezepelumab: for severe asthma when treatment with high-dose ICS plus another maintenance treatment has not controlled symptoms, only if:[122]National Institute for Health and Care Excellence. Tezepelumab for treating severe asthma. Apr 2023 [internet publication].
https://www.nice.org.uk/guidance/ta880
[123]Menzies-Gow A, Wechsler ME, Brightling CE, et al. Long-term safety and efficacy of tezepelumab in people with severe, uncontrolled asthma (DESTINATION): a randomised, placebo-controlled extension study. Lancet Respir Med. 2023 May;11(5):425-38.
http://www.ncbi.nlm.nih.gov/pubmed/36702146?tool=bestpractice.com
The patient has had 3 or more exacerbations in the previous year, or
The patient is taking maintenance oral corticosteroids.
Some biologics are suitable for self-administration at home after appropriate training.[124]Asthma and Lung UK. Biologic therapies for severe asthma. Dec 2021 [internet publication].
https://www.asthmaandlung.org.uk/symptoms-tests-treatments/treatments/biologic-therapies
Bronchial thermoplasty
This bronchoscopic procedure aims to reduce bronchial smooth muscle mass, therefore reducing the capacity for bronchoconstriction. In the UK, only a few specialist centres offer this treatment, which has considerable resource implications.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
Any patients being considered for bronchial thermoplasty should be assessed to confirm the diagnosis of asthma, that uncontrolled asthma is the cause of their ongoing symptoms, and that they are adherent with current treatment.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
An asthma specialist with expertise in bronchial thermoplasty should assess patients prior to undergoing treatment, and treatment should take place in a specialist centre with the appropriate resources and training, including access to an intensive care unit.[55]British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline. Jul 2019 [internet publication].
https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma
In people with severe asthma, bronchial thermoplasty improves asthma-specific quality of life, with a reduction in severe exacerbations and healthcare use in the post-treatment period.[125]National Institute for Health and Care Excellence. Bronchial thermoplasty for severe asthma. Dec 2018 [internet publication].
https://www.nice.org.uk/guidance/ipg635
[126]Torrego A, Solà I, Munoz AM, et al. Bronchial thermoplasty for moderate or severe persistent asthma in adults. Cochrane Database Syst Rev. 2014 Mar 3;(3):CD009910.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009910.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/24585221?tool=bestpractice.com
In the UK, patients undergoing bronchial thermoplasty should have their details entered onto the Severe Asthma Registry. Bronchial thermoplasty is an invasive procedure and is associated with a high rate of adverse respiratory events in the short term.[127]Zhou JP, Feng Y, Wang Q, et al. Long-term efficacy and safety of bronchial thermoplasty in patients with moderate-to-severe persistent asthma: a systemic review and meta-analysis. J Asthma. 2016;53(1):94-100.
http://www.ncbi.nlm.nih.gov/pubmed/26383773?tool=bestpractice.com
NICE
Although the evidence base informing the BTS/SIGN and NICE guidelines was broadly the same, the methodologies employed by the two guideline development groups were substantially different, leading to discrepancies in the recommendations for management of asthma. NICE's approach is summarised in the flowchart below and the text that follows.
[Figure caption and citation for the preceding image starts]: Pharmacological treatment of chronic asthma in adults aged 17 and over; reproduced from NICE “Asthma: diagnosis, monitoring and chronic asthma management”National Institute for Health and Care Excellence. Algorithm F: Pharmacological treatment of chronic asthma in adults aged 17 and over. 2017 [internet publication]; used with permission [Citation ends].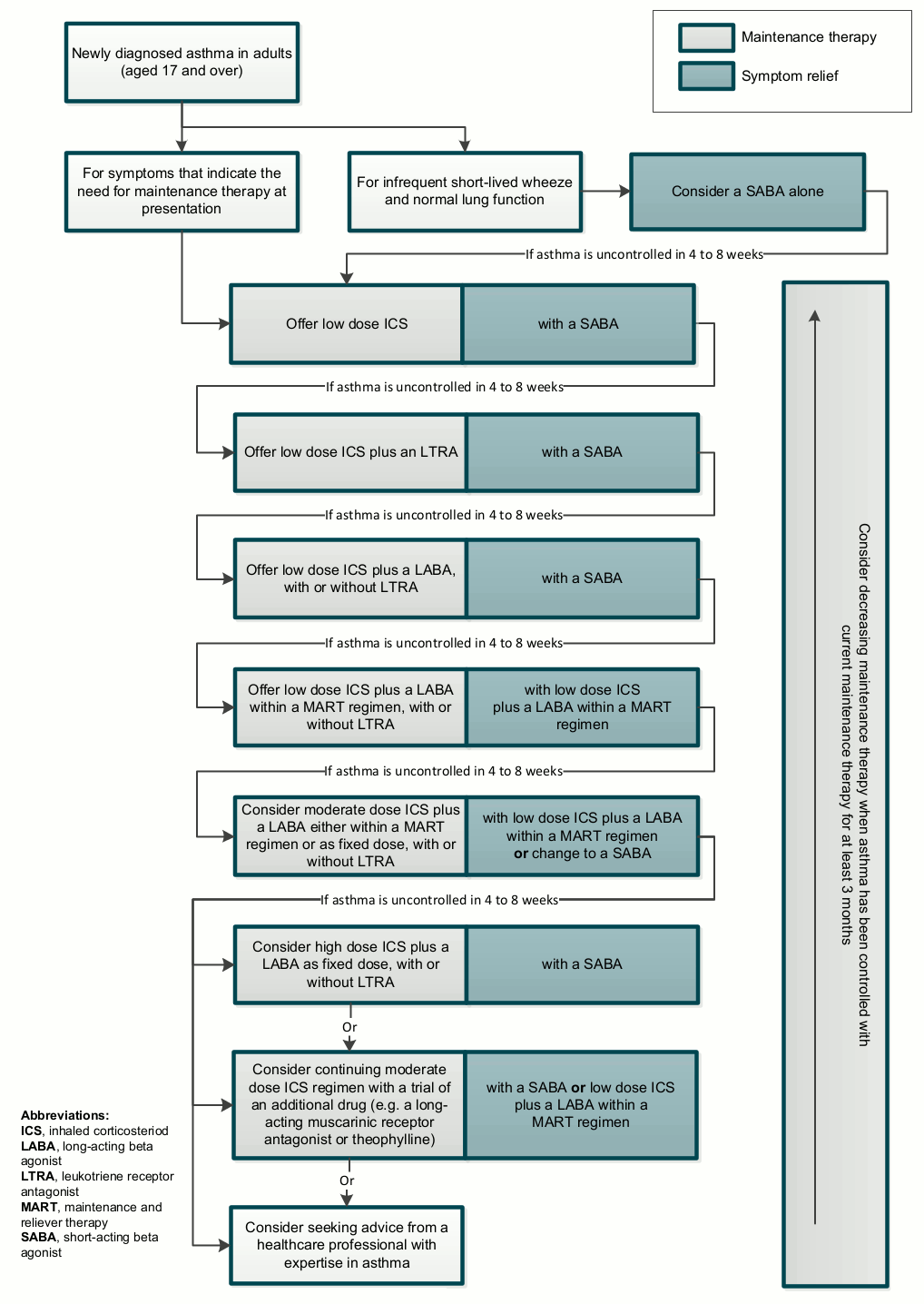
Step 1 - initial therapy for control of asthma
NICE advocates the use of SABA reliever therapy alone, to be used as needed to relieve symptoms in symptomatic patients if the patient has infrequent, short-lived wheeze and normal lung function.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
NICE recommends to consider adding low-dose ICS to SABA if the patient has any of the following asthma-related features:[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Acute asthma attack (requiring oral corticosteroids) in the past 2 years
Using inhaled SABA three times a week or more
Symptomatic three times a week or more
Waking one night a week.
Step 2 - initial add-on therapy
If asthma is uncontrolled in adults on low-dose ICS as preventer therapy, NICE recommends an LTRA in addition to the ICS; review the response to treatment in 4 to 8 weeks.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
If the patient’s symptoms remain uncontrolled on a low dose of ICS and an LTRA as preventer therapy, NICE recommends to give a LABA in combination with the ICS and review LTRA treatment by:[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
NICE recommends that the patient can continue to use intermittent reliever therapy (usually an inhaled SABA) as needed throughout all the steps outlined above.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
If symptoms remain uncontrolled on a low dose of ICS and a LABA (with or without an LTRA), NICE recommends to consider changing the patient's ICS and LABA maintenance therapy to a low-dose LABA and ICS MART regimen.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
NICE states that the patient should continue with an LTRA alongside a MART regimen if they had a response to the LTRA at the previous step.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
NICE advises that the patient should not use intermittent short-acting reliever therapy (e.g., an inhaled SABA) alongside MART.[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Step 3 - additional controller therapies
If the patient's symptoms remain uncontrolled on a MART regimen with a low-dose LABA/ICS, with or without an LTRA, NICE recommends to consider increasing the ICS dose to medium (either continuing on a MART regimen or changing to a fixed dose of an ICS and a LABA, with a SABA as a reliever therapy).[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Step 4 - specialist therapies
If the patient's symptoms remain uncontrolled despite a medium maintenance ICS dose with a LABA (either as MART or a fixed-dose regimen), with or without an LTRA, NICE recommends to consider one of the following approaches:[56]National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. Mar 2021 [internet publication].
https://www.nice.org.uk/guidance/ng80
Increasing the ICS to a high maintenance dose (this should only be offered as part of a fixed-dose regimen, with a SABA used as a reliever therapy)
A trial of an additional drug (e.g., a LAMA or theophylline)
Seeking advice from a healthcare professional with expertise in asthma.
 ]
This should be supported by regular professional review.[55][56] Advise the patient to take environmental control measures (e.g., reduce exposure to indoor and outdoor air pollution, tobacco smoke, and occupational and domestic allergens).[1][55][56] If you suspect an occupational cause, see Occupational asthma.
]
This should be supported by regular professional review.[55][56] Advise the patient to take environmental control measures (e.g., reduce exposure to indoor and outdoor air pollution, tobacco smoke, and occupational and domestic allergens).[1][55][56] If you suspect an occupational cause, see Occupational asthma. ]
]
 ]
]
 ]
]
 ]
In the UK, NICE recommends allowing 4 to 8 weeks to assess the patient's response before making a decision on whether a step up to the next level of treatment is needed.[96]
]
In the UK, NICE recommends allowing 4 to 8 weeks to assess the patient's response before making a decision on whether a step up to the next level of treatment is needed.[96]
 ]
]
