History and exam
Your Organisational Guidance
ebpracticenet urges you to prioritise the following organisational guidance:
Multidisciplinaire richtlijn Postpartumzorg in de eerste lijn (deel 1)Published by: Werkgroep Ontwikkeling Richtlijnen Eerste Lijn (Worel)Last published: 2022Guideline multidisciplinaire des soins postnatals dans la première ligne de soins (partie 1)Published by: Groupe de Travail Développement de recommmandations de première ligneLast published: 2022Multidisciplinaire richtlijn Postpartumzorg in de eerste lijn (deel 2)Published by: Werkgroep Ontwikkeling Richtlijnen Eerste Lijn (Worel)Last published: 2024Guideline multidisciplinaire des soins postnatals dans la première ligne de soins (partie 2)Published by: Groupe de Travail Développement de recommmandations de première ligneLast published: 2024Key diagnostic factors
common
estimated blood loss (EBL) ≥500 mL within 24 hours of birth
Make a clinical diagnosis of primary postpartum haemorrhage (PPH) based on an EBL ≥500 mL within 24 hours of birth. Categorise PPH as minor (EBL 500-1000 mL) or major (EBL >1000 mL). Major PPH may be moderate (EBL 1000-2000 mL) or severe (EBL >2000 mL).[4][5][36]
Note that the degree of EBL that warrants a diagnosis of primary PPH varies between guidelines. Check your local protocol. See Diagnosis approach.
Be aware that visual estimation can result in underestimation of blood loss by 33% to 50%, particularly when large volumes are lost.[52] Ensure your assessment of PPH incorporates both EBL and clinical signs/symptoms of blood loss. Do not rely solely on either alone.[3][4][70] Use an objective method to measure blood loss (e.g., by weighing blood-soaked drapes or surgical sponges).[71]
uterine atony
Diagnosed based on a soft, poorly contracted (boggy) palpable uterus above the level of the umbilicus despite uterine massage on bimanual examination.[3]
The most common cause of postpartum haemorrhage, accounting for approximately 70% to 80% of cases.[3][20] It can be associated with intrapartum infection (chorioamnionitis or endometritis).[21]
obstetric lacerations and/or expanding haematomas
Obstetric trauma is the second most common cause of postpartum haemorrhage (PPH), accounting for 20% to 30% of cases.[3][20] It is therefore essential to perform a thorough examination of the abdomen and pelvis that allows for proper visualisation of the cervix and the entire length of the vaginal walls.[36]
A detailed pelvic examination is required to identify unrepaired cervical, sulcal, or perineal lacerations, as well as expanding pelvic haematomas. Early intervention is paramount to prevent worsening of PPH.
Note that PPH resulting from some forms of trauma (e.g., cervical or high vaginal lacerations) may only be detected during an examination under anaesthesia in the operating room.[83]
signs of hypovolaemia
Ensure your assessment of postpartum haemorrhage (PPH) incorporates both estimated blood loss (EBL) and clinical signs/symptoms of blood loss. Do not rely solely on either alone.[3][4][36][70]
Signs of acute blood loss include: hypotension (systolic blood pressure [SBP] <90 mmHg); tachycardia (heart rate >100 bpm); tachypnoea (respiratory rate >20 breaths per minute); decreased urine output; and decreased pulse pressure (the difference between systolic and diastolic pressures: normally 35-45 mmHg).[3][6][72]
Be aware that vital sign derangements are often a late manifestation of significant blood volume loss and hypovolaemic shock.[73][74] The association between vital sign thresholds and degree of haemorrhage is more variable in obstetric patients compared with the general adult population because of the physiological increase in circulating blood volume.[4] In pregnancy, traditional signs of hypovolaemia such as heart rate and blood pressure are usually maintained in the normal range until blood loss exceeds 1000 mL; tachycardia, tachypnoea, and a slight fall in SBP occur with blood loss of 1000-1500 mL; a SBP <80 mmHg, associated with worsening tachycardia, tachypnoea, and altered mental state, usually indicates a PPH >1500 mL.[4]
The shock index (SI) can serve as a predictive indicator of haemodynamic changes resulting from acute blood loss, even when blood pressures remain within the normal range.[4][74][79][80] The SI is calculated by dividing the maternal heart rate by the SBP. SI values above 0.9 have been associated with an increased risk of requiring massive transfusion, admission to the intensive care unit, and adverse outcomes related to PPH.
uncommon
retained tissue (placenta, membranes, or placenta accreta spectrum)
Retained products of conception is a complication affecting approximately 2% to 3% of all births and is the cause of around 10% of cases of postpartum haemorrhage.[3][20][84] Placenta accreta spectrum (PAS) disorders include placenta accreta, increta, or percreta.
Retained placental tissue is diagnosed with careful bimanual examination upon postpartum palpation of placental tissue or membranes within the uterine cavity and can be confirmed by bedside ultrasound.[3]
Inspect the placenta itself to ensure intact placental delivery, and to assess for the presence of missing placental tissue or membranes.[3][21]
[Figure caption and citation for the preceding image starts]: Ultrasound image showing retained product of conception within the endometrial cavity (measuring 20 x 8mm) in a woman with secondary PPH. The image was taken on day 22 post-deliveryDu R, et al. BMJ Case Reports CP 2021;14:e245009; used with permission [Citation ends].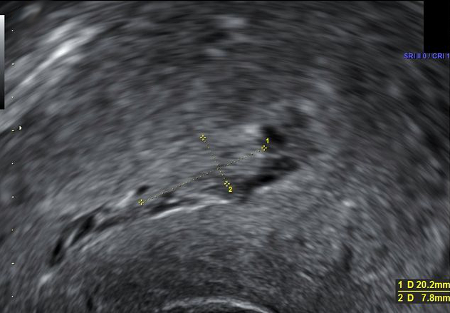
uterine defect on bimanual examination
Uterine rupture may be diagnosed through palpation of a uterine defect on bimanual examination. The most common prior intrapartum symptoms associated with uterine rupture include acute-onset persistent abdominal pain, vaginal bleeding, tachysystole on tocometry, loss of fetal station, and/or a non-reassuring fetal heart rate tracing in the setting of a prior uterine scar.[81]
If the uterine fundus cannot be palpated during a postpartum haemorrhage, consider evaluating for uterine inversion, as incomplete or partial uterine inversion may not be immediately recognised on initial examination. A mass may or may not be palpated within the uterus, or visualised protruding from the vagina, representing the uterine fundus. These patients may experience significant shock or hypotension out of proportion to the degree of blood loss, due to vasovagal stimulation.[82]
Other diagnostic factors
common
symptoms of hypovolaemia
Symptoms of acute blood loss may include dizziness, pallor, and altered mental state.[6]
uncommon
uterine tenderness (secondary postpartum haemorrhage [PPH])
signs of infection (secondary postpartum haemorrhage [PPH])
Risk factors
strong
placenta previa/low lying placenta
Placenta previa is associated with a high risk of PPH, due to the potential for bleeding during delivery. One systematic review found that between 16% and 29% of patients with placenta previa or low lying placenta experience PPH.[23]
Patients with placenta previa have been found to have a 9- to 16-fold increased risk of PPH compared with those without placenta previa.[24][25]
placenta accreta spectrum
Placenta accreta spectrum (PAS) disorders - including placenta accreta, increta, and percreta - significantly increase the risk of PPH due to retained placental tissue from abnormal placental invasion, which can result in catastrophic haemorrhage.
PAS has been identified as the most common cause of massive bleeds (whereas uterine atony is the most common cause of moderately severe bleeds), accounting for 6% to 7% of maternal mortality rates in the US.[26][27][28] PAS disorder has been associated with up to an 8-fold increased risk of severe PPH.[24]
PAS disorders comprise: placenta accreta (70% of cases), where the placenta is abnormally adherent to the uterine myometrium; placenta increta, where the placenta invades into the uterine myometrium; and placenta percreta, where the placenta invades through the uterine myometrium, serosa, and even possibly to surrounding organs. It is often associated with prior uterine surgery.[21]
The incidence of PAS has continued to increase significantly with increasing rates of caesarean delivery.[29]
Antenatal diagnosis of PAS disorders with obstetric ultrasound or MRI is extremely useful for surgical planning. See Primary prevention.
platelet count <50 × 10⁹/L (<50,000 per microlitre)
Significant thrombocytopenia is associated with a high risk of PPH, whether from immune thrombocytopenia (ITP) or other acquired platelet dysfunction disorders, such as pre-eclampsia/HELLP syndrome.
Women with a platelet count <50 × 10⁹/L (<50,000 per microlitre) appear to be at highest risk for PPH compared with women with normal platelet counts (odds ratio 2.24, 95% CI 1.01 to 4.94).[30]
See Pre-eclampsia, HELLP syndrome.
active antepartum bleeding
Patients who present to labour and delivery with active bleeding are at higher risk for PPH due to the risk of continued bleeding postpartum, although the incidence varies based on the underlying aetiology of active bleeding and specific clinical presentation.
In one study, antepartum haemorrhage was associated with a significantly increased risk of uterine atony attributing to PPH (odds ratio 3.8, 95% CI 3.0 to 4.8).[31]
inherited coagulopathy
Women with inherited bleeding disorders such as thrombophilias, von Willebrand disease (VWD), haemophilia, factor XI deficiency, and other rare coagulopathies have been shown to have a higher incidence of PPH.
Specifically, women with VWD have been demonstrated to have up to a 3-fold increased risk of PPH compared with those without VWD.[32][33][34]
acquired coagulopathy
Acquired coagulopathies are associated with an increased risk of PPH from the development of disseminated intravascular coagulation (DIC) and maternal coagulopathy. Evidence shows that even mild haemostatic abnormalities such as low levels of fibrinogen are independently associated with a significantly increased risk of PPH.[35]
Maternal coagulopathy may be triggered by pre-eclampsia, HELLP syndrome, intrauterine fetal demise, placental abruption, amniotic fluid embolism, dilution in the setting of significant PPH, or the use of therapeutic anticoagulation.
history of PPH in prior delivery
History of PPH in a prior delivery is associated with a moderately increased risk of PPH in a subsequent pregnancy.
Patients with a history of PPH in a prior pregnancy have been found to have a 3-fold increased risk of recurrent PPH in a subsequent pregnancy compared with women with no prior history of PPH.[36] This risk increased with increasing numbers of previously affected pregnancies.[37]
History of severe PPH in a prior pregnancy has been associated with up to an 8- to 9-fold increased risk of recurrent PPH in one study.[38]
operative (assisted) vaginal delivery
Operative vaginal delivery (with forceps or ventouse) can increase the risk for obstetric lacerations and subsequent PPH.[21]
In one large study, operative vaginal delivery was associated with a 2- to 9-fold increased risk of severe PPH when compared with spontaneous vaginal delivery, with the risk highest for multiparous women (second or subsequent birth).[39]
current use of therapeutic anticoagulation
Current anticoagulation use has been associated with a 4- to 5-fold increased risk of PPH.[38]
weak
prior caesarean delivery, uterine surgery, or multiple laparotomies
Prior uterine surgery is associated with a moderately increased risk for PPH due to the risk of repeat caesarean delivery or abnormal placentation.[21] History of a previous caesarean section has been associated with a 2- to 3-fold increased risk of PPH in one population-based cohort study and was classified as a medium risk factor in a retrospective cohort study of 10,134 women.[24][40]
uterine overdistension (multiple gestation, polyhydramnios, fetal macrosomia with estimated fetal weight >4000 g)
Uterine overdistension is associated with a moderately increased risk for PPH due to uterine atony.[21] Fetal macrosomia has been associated with a 3- to 4-fold increased risk of PPH, while polyhydramnios has been associated with a 1- to 2-fold increased risk of uterine atony contributing to PPH.[41] Meanwhile, multiple gestation has been associated with a 4- to 5-fold increased risk of PPH.[41]
grand multiparity (>4 prior births)
large uterine myomas
class III obesity (BMI >40)
Class III obesity (BMI >40 kg/m²) is associated with a moderately increased risk for PPH due to risk of uterine atony. In one study, obesity was associated with an increased risk for PPH following caesarean delivery (odds ratio [OR] 1.73, 95% CI 1.32 to 2.28) as well as vaginal delivery (OR 2.11, 95% CI 1.54 to 2.89).[43]
pre-existing maternal anaemia
Pre-existing anaemia on admission (haematocrit <30%) is associated with an increased risk for PPH and worsening anaemia. Pre-delivery anaemia has been associated with a 2- to 4-fold increased risk for severe PPH.[24][38]
Cohort analysis of data from the WOMAN-2 trial found that the risk of death or near-miss from PPH was 7 times higher for severe anaemia (haemoglobin [Hb] <70 g/L) compared with moderate anaemia (Hb 70-99 g/L).[44]
prolonged labour or precipitous delivery
Prolonged labour increases the risk for PPH due to uterine atony, while precipitous labour may increase the risk for PPH due to obstetric lacerations and/or uterine atony.[21]
Prolonged active labour (>12 hours) was found in one study to be associated with a 2- to 3-fold increased risk of severe PPH.[45] Other studies have shown up to an overall 5-fold increased risk of PPH with prolonged labour.[24]
labour induction or augmentation with prolonged use of oxytocin
magnesium sulfate use
caesarean delivery
Caesarean delivery is associated with a higher risk of PPH than vaginal delivery, especially in the setting of emergency caesarean delivery.
Risk of PPH has been shown to be 1- to 2-fold higher in women undergoing caesarean delivery compared with spontaneous labour.[31]
placental abruption
Placental abruption has been associated with a 2- to 4-fold increased risk of PPH.[24][25] Abruption can be associated with uterine atony due to the extravasation of blood into the myometrium (a Couvelaire uterus), resulting in disseminated intravascular coagulation (DIC) and hypofibrinogenaemia.[3]
Placental abruption accounts for 17% of cases of PPH requiring massive transfusion.[47] It usually presents as a combination of vaginal bleeding, frequent uterine contractions, and pain. The classic contraction pattern includes high-frequency, low-amplitude contractions.[3]
severe pre-eclampsia or HELLP syndrome
intrauterine fetal demise (IUFD)
IUFD can increase the risk of PPH from the development of disseminated intravascular coagulation (DIC) and maternal coagulopathy. Stillbirth has been associated with a 2- to 3-fold increased risk of PPH.[24]
intrapartum infection
Chorioamnionitis or endometritis can increase the risk of PPH through uterine inflammation, resulting in impaired contractility and uterine atony, as well as coagulopathy in severe cases.[21]
Chorioamnionitis has been associated with a 2- to 3-fold increased risk of PPH at the time of caesarean delivery.[25]
selective serotonin-reuptake inhibitor (SSRI) or serotonin-noradrenaline reuptake inhibitor (SNRI) use in the month before delivery
The UK Medicines and Healthcare products Regulatory Agency (MHRA) warns of a small increased risk of PPH in women who have used an SSRI or SNRI antidepressant in the month before delivery.[48] This increased risk is attributable to the known increased bleeding risks from SSRIs and SNRIs, owing to their serotonergic effect in impairing platelet aggregation.
The warning followed a review by the European Medicines Agency (EMA) which identified observational studies that reported an increased risk of PPH associated with antidepressant use in late pregnancy, particularly for SSRIs and SNRIs.[49] The increased risk for use of an antidepressant in the month before delivery was estimated to be less than 2-fold.[48]
Use of this content is subject to our disclaimer