Recommendations
Your Organisational Guidance
ebpracticenet urges you to prioritise the following organisational guidance:
Behandeling acuut coronair syndroom in een urgente situatie (in afwachting van hospitalisatie)Published by: Werkgroep Ontwikkeling Richtlijnen Eerste Lijn (Worel)Last published: 2022La prise en charge du syndrome coronarien aigu (SCA) en situation d'urgence (en attente d'hospilatisation)Published by: Groupe de Travail Développement de recommmandations de première ligneLast published: 2022Urgent
Record and interpret a resting 12-lead ECG within 10 minutes of the point of first medical contact in any patient with suspected NSTEMI (and for other acute coronary syndromes [ACS]).[5]
Discuss the patient immediately with the cardiology team and involve senior support if the ECG shows evidence of ST-elevation myocardial infarction (STEMI) to activate your local STEMI protocol. See ST-elevation myocardial infarction.[72]
Be aware that the ECG may be normal in more than 30% of patients.[5]
Make an initial working clinical diagnosis of non-ST-elevation acute coronary syndrome (NSTE-ACS) based on the presence of symptoms suggestive of myocardial ischaemia (e.g., chest pain) and ECG findings (no evidence of STEMI).
Arrange troponin testing; a dynamic elevation of cardiac troponin above the 99th percentile indicates myocardial infarction and confirms a diagnosis of NSTEMI.[5]
Organise urgent echocardiography for any patient with signs of acute heart failure or haemodynamic instability or who is in cardiac arrest.[5] This should only be performed by those with specialist training.
Get urgent input from a senior colleague or cardiology if the patient is clinically unstable or has any very high-risk features (as outlined below) to arrange immediate invasive coronary angiography (with the intent to perform revascularisation). Do not wait for the results of troponin testing.[5] This includes any patient with:[5][72][73]
Ongoing or recurrent pain despite treatment
Haemodynamic instability (low blood pressure or shock) or cardiogenic shock; see Shock
Recurrent dynamic ECG changes
Acute left ventricular failure; see Acute heart failure
A life-threatening arrhythmia (ventricular tachycardia or ventricular fibrillation) or cardiac arrest after presentation; see Sustained ventricular tachycardias
Mechanical complications such as new-onset mitral regurgitation.
In the community, refer the patient to hospital as an emergency if you suspect ACS and they:[74]
Currently have chest pain
Are currently pain free, but have had chest pain within the last 12 hours and a resting 12-lead ECG is abnormal or unavailable
Have had a recent ACS (confirmed or suspected) and develop further chest pain.
Key Recommendations
Consider ACS in any patient presenting with chest pain, which includes other areas (e.g., the arms, back, or jaw), especially if this is associated with nausea and vomiting, marked sweating, and/or breathlessness, or particularly a combination of these.[74]
Recognise that presentations where chest pain is not the predominant feature (chest-pain equivalent symptoms) are more common in older patients, women, and patients with diabetes.[5] These include epigastric pain, indigestion-like symptoms, isolated dyspnoea, or syncope.[5] Women are also more likely to present with middle/upper back pain.
Take into account the history and character of the chest pain, risk factors for cardiovascular disease (particularly a history of ischaemic heart disease) and any previous treatment, and previous investigations for chest pain to determine if ACS is likely.[74]
Be aware that physical examination may be normal.[1] However, important signs to look out for are new murmurs and marked sweating.[5][74]
Always order the following, in addition to a resting 12-lead ECG, for all patients:
High-sensitivity troponin (within 60 minutes); use this in conjunction with a diagnostic algorithm to rapidly confirm or rule out NSTEMI (a summary of the pathway recommended by the European Society of Cardiology [ESC] is below).[5][75]
Chest x-ray
Full blood count
Urea, electrolytes, and creatinine
Liver function tests
Blood glucose
C-reactive protein.
The ESC recommends that patients are classified into one of three pathways as per the results of their high-sensitivity cardiac troponin (hs-cTn) values at 0 hours (time of initial blood test) and 1 hour or 2 hours later.
Rule-out pathway: for very low initial hs-cTn or no increase after 1/2 hours: these patients may be appropriate for early discharge and outpatient management.
Rule-in pathway: for high initial hs-cTN or an increase after 1-2 hours: most of these patients will require hospital admission and invasive coronary angiography.
Observe pathway: if neither of the above criteria is met: check hs-cTN at 3 hours and consider echocardiography.
In practice, patients with a raised hs-cTN presenting in the accident and emergency department are often referred to cardiology before a second troponin test.
Get urgent input from a senior colleague or cardiology if the patient is clinically unstable or has any very high-risk features (as outlined below) to arrange immediate invasive coronary angiography (with the intent to perform revascularisation). This includes any patient with:[72][73]
Ongoing or recurrent pain despite treatment
Haemodynamic instability (low blood pressure or shock); see Shock
Dynamic ECG changes
Left ventricular failure; see Acute heart failure
A life-threatening arrhythmia (ventricular tachycardia or ventricular fibrillation) or cardiac arrest after presentation; see Sustained ventricular tachycardias[5]
Mechanical complications such as new-onset mitral regurgitation.[5]
Make an initial working clinical diagnosis of NSTE-ACS based on the presence of symptoms suggestive of myocardial ischaemia (e.g., chest pain) and ECG findings (no evidence of STEMI).
Arrange troponin testing; a dynamic elevation of cardiac troponin above the 99th percentile indicates myocardial infarction and confirms a diagnosis of NSTEMI.[5] If dynamic troponin testing shows cardiac troponin remaining below the 99th percentile, this usually suggests a diagnosis of unstable angina. See Unstable angina.
Suspect NSTEMI (and other ACS) in any patient presenting with chest pain, which includes pain in other areas (e.g., the arms, back, or jaw), that:[74]
Lasts longer than 15 minutes
Is associated with nausea and vomiting, marked sweating, and/or breathlessness, or particularly a combination of these[74]
Is associated with haemodynamic instability
Is either new in onset or occurs as sudden worsening of known stable angina (i.e., recurrent episodes of chest pain lasting longer than 15 minutes that occur frequently and with little or no exertion).
Practical tip
Patients may also describe chest pain as pressure, tightness, heaviness, or a burning sensation.[5]
Be aware of non-characteristic presentations such as epigastric pain, indigestion-like symptoms, isolated dyspnoea, or syncope. These are more common in older patients, women, and patients with diabetes.[5] Women are also more likely to present with middle/upper back pain and other non-characteristic symptoms.
In the community, refer all patients to hospital as an emergency if you suspect an ACS and they:[74]
Currently have chest pain
Are currently pain free, but have had chest pain within the last 12 hours and a resting 12-lead ECG is abnormal or unavailable
Have had a recent ACS (confirmed or suspected) and develop further chest pain.
Practical tip
The European Society of Cardiology recommends to ' Think A.C.S' at initial assessment of chest pain:[5]
Abnormalities or evidence of ischaemia on ECG assessment
Clinical context: take a targeted clinical history to assess the clinical context of the presentation
Stability: targeted clinical examination to assess for clinical and haemodynamic stability.
The admitting team can decide whether immediate invasive management is required based on this initial assessment.
Check immediately if the patient currently has chest pain.[74]
Consider the following to determine whether the chest pain is likely to be cardiac:[74]
The history and character of the patient’s chest pain.[5][74] Useful points to cover include:
Whether the patient has experienced this type of pain before
The nature, severity, and duration of pain
Often a retrosternal sensation of pain, pressure, or heaviness radiating to the left arm, both arms, right arm, neck, or jaw, which may be intermittent or persistent.[5]
Ask when the patient’s chest pain started because this will determine the timing and interpretation of high-sensitivity troponin testing.[74]
If symptoms are intermittent, it is important to ask when the last episode of pain occurred.[74]
Any associated symptoms.
The presence of risk factors for cardiovascular disease.[1][74][76] These include:
Diabetes
Hyperlipidaemia
Hypertension
Metabolic syndrome
Renal impairment
Peripheral arterial disease
A history of ischaemic heart disease and any previous treatment
Obesity
Advanced age
Smoking
Cocaine use
Physical inactivity
Family history of premature coronary artery disease (<60 years).
Previous investigations for chest pain.[74]
Ask the patient about other important points:
Get urgent input from a senior colleague or cardiology if the patient is haemodynamically unstable (low blood pressure or shock), has evidence of left ventricular failure, or has a life-threatening arrhythmia (VT or VF) to arrange immediate invasive coronary angiography (with the intent to perform revascularisation).[72][73] See Shock, Acute heart failure, and Sustained ventricular tachycardias.
Look out for other signs such as:
A new murmur
A systolic murmur may be present due to ischaemic mitral regurgitation, which is associated with a poor prognosis, or a mechanical complication of NSTEMI (e.g., papillary muscle rupture or ventricular septal defect).
Significant sweating.[74]
Assess for any signs of active bleeding.
Practical tip
Remain vigilant for alternative diagnoses.[78]
Examine for signs of aortic dissection (such as radio-radial delay); this is an important differential to consider. See Aortic dissection.
Fever may be suggestive of endocarditis or pneumonia.
Auscultate the lungs for abnormal lung sounds; this may reveal signs of pneumonia or pneumothorax.
Auscultate the heart for added sounds: a friction rub may suggest pericarditis; other murmurs may reveal signs suggestive of valvular stenosis or regurgitation, or endocarditis.
Immediate (within 10 minutes) in all patients
ECG
Record and interpret a resting 12-lead ECG within 10 minutes of the point of first medical contact in any patient with suspected cardiac chest pain.[5]
Discuss the patient immediately with the cardiology team and involve senior support if the ECG shows evidence of STEMI to activate your local STEMI protocol.[5] See ST-elevation myocardial infarction.
Abnormal findings that suggest NSTEMI include:[1][5]
ST depression; this indicates a worse prognosis
Transient ST elevation
T-wave changes.
Be aware that the ECG may be normal in more than 30% of patients.[5]
Record additional leads if the standard leads are inconclusive, if total vessel occlusion is suspected, or in cases of suspected inferior STEMI.[5]
Leads V 7-V 9 may detect left circumflex artery occlusion and leads V 3R and V 4R may detect right ventricular myocardial infarction.[5]
Order repeated resting ECGs if the patient has recurrent symptoms or you are unsure about the diagnosis.[5][74]
Always compare the current ECG with previous ECGs where possible.[74]
How to record an ECG. Demonstrates placement of chest and limb electrodes.
[Figure caption and citation for the preceding image starts]: Position of ECG leads V7-V9Image used with permission from BMJ 2002;324:831 [Citation ends].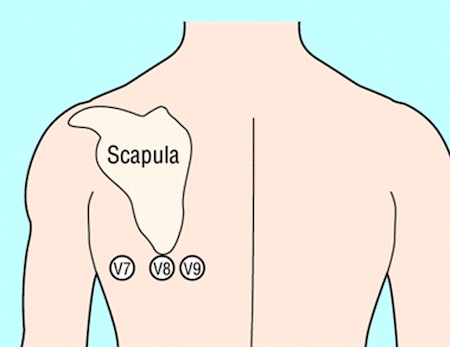 [Figure caption and citation for the preceding image starts]: Position of right precordial ECG leads V3R and V4RImage used with permission from BMJ 2002;324:831 [Citation ends].
[Figure caption and citation for the preceding image starts]: Position of right precordial ECG leads V3R and V4RImage used with permission from BMJ 2002;324:831 [Citation ends].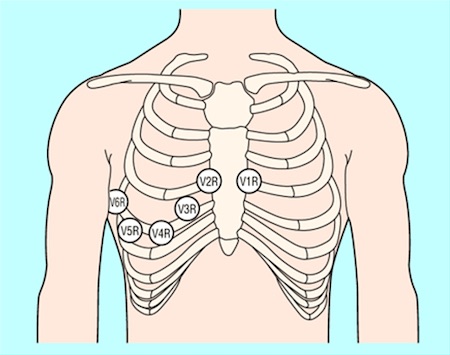 [Figure caption and citation for the preceding image starts]: ECG showing inferolateral ST depressionFrom the personal collection of Dr Syed W. Yusuf and Dr Iyad N. Daher, Department of Cardiology, University of Texas, Houston; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: ECG showing inferolateral ST depressionFrom the personal collection of Dr Syed W. Yusuf and Dr Iyad N. Daher, Department of Cardiology, University of Texas, Houston; used with permission [Citation ends].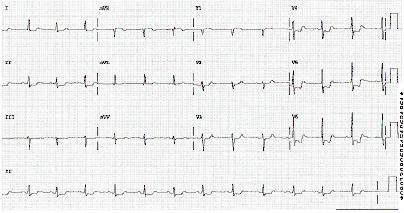 [Figure caption and citation for the preceding image starts]: ECG showing inferolateral ST depressionFrom the personal collection of Dr Syed W. Yusuf and Dr Iyad N. Daher, Department of Cardiology, University of Texas, Houston; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: ECG showing inferolateral ST depressionFrom the personal collection of Dr Syed W. Yusuf and Dr Iyad N. Daher, Department of Cardiology, University of Texas, Houston; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: ECG showing T wave inversion in leads V1-V4, III and aVF.BMJ Learning/Professor Kevin Tanner; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: ECG showing T wave inversion in leads V1-V4, III and aVF.BMJ Learning/Professor Kevin Tanner; used with permission [Citation ends].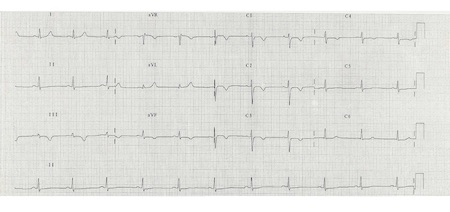
Acute (within 60 minutes) in all patients
High-sensitivity troponin
Measure high-sensitivity cardiac troponin (hs-cTn) immediately after presentation and obtain results within 60 minutes in all patients with suspected ACS.[5]
Use a diagnostic algorithm: the ESC recommends using the 0/1 hour or 0/2 hour 'rule in' and 'rule out' algorithms, which classify patients into one of three pathways according to the results of their hs-cTn values at 0 hours (time of initial blood test) and 1 hour or 2 hours later.[5]
Rule-out pathway: for very low initial hs-cTn or no increase after 1/2 hours: these patients may be appropriate for early discharge and outpatient management.
Rule-in pathway: for high initial hs-cTN or an increase after 1-2 hours: most of these patients will require hospital admission and invasive coronary angiography.
Observe pathway: if neither of the above criteria is met: check hs-cTN at 3 hours and consider echocardiography.
In practice, patients with a raised hs-cTN presenting in the accident and emergency department are often referred to cardiology before a second troponin test.
Cut-off values for hs-cTn are dependent on the assay used; check your local protocol.[5]
Use the algorithm in the context of other clinical criteria such as a detailed history of the chest pain and any ECG findings.[5]
Some patients may need an additional measurement of troponin at 3 hours (e.g., if the first two hs-cTn measurements of the 0/1 algorithm are inconclusive, and no alternative diagnoses explaining the condition have been made.[5]
The higher the hs-cTn at presentation, the higher the risk of death.[5]
Practical tip
Reassess the patient if they have a raised hs-cTn.
Troponin can be acutely raised due to other causes such as myocarditis, aortic dissection, or acute pulmonary embolism.[74]
Chronic elevation of high-sensitivity troponin is also common in patients with renal dysfunction and heart failure.[1][5]
Troponin levels may remain elevated for 1-2 weeks following recent myocardial infarction and/or percutaneous coronary intervention.
Interpret hs-cTn in the context of the clinical scenario.
Evidence: High-sensitivity troponin tests in early rule-out protocols
Use of high-sensitivity troponin allows for a rapid ‘rule-out’ of NSTEMI using an accelerated diagnostic algorithm (e.g., high-sensitivity troponin at 0 and 1 hours).
The 2023 European Society of Cardiology (ESC) guideline for the management of acute coronary syndromes recommends using a 0 and 1 hour rapid 'rule out' and 'rule in' protocol if a high-sensitivity troponin assay is available.[5]
This was based on evidence of a reduced delay to diagnosis, which has been shown to decrease accident and emergency department stay and lower costs through identification of patients who can be discharged early and managed as outpatients. The second best option was a 0 and 2 hour algorithm.
The very high safety and high efficacy of the ESC 0 and 1 hour algorithm has been confirmed in three real-life implementation studies (RAPID-CPU, High-STEACS, and RAPID-TnT), of which one, RAPID-TnT, was a randomised controlled trial.[79][80][81]
Both algorithms were developed in large derivation cohorts and then validated in large independent cohorts.
The guideline panel noted that these algorithms should always be integrated into a clinical pathway with clinical assessment and 12-lead ECG, and that repeat blood sampling is mandatory if the patient has ongoing or recurrent chest pain.
The ESC guideline panel selected optimal thresholds for rule-out to allow for a minimal sensitivity and negative predictive value (NPV) for myocardial infarction of 99%; while optimal thresholds for rule-in allowed for a minimal positive predictive value (PPV) for myocardial infarction of 70%.[5][82]
The 2015 ESC guideline had recommended a 0 and 3 hour algorithm. However, three subsequent large diagnostic studies suggested this algorithm performed less well in terms of efficacy and safety compared with more rapid protocols using lower rule-out concentrations.[80][83][84]
However, the 2023 guideline noted that with the rapid protocols the following patients may need additional cardiac troponin concentration at 3 hours:
If the first two hs-cTn measurements of the 0/1 algorithm are inconclusive and no alternative diagnoses explaining the condition have been made.
In 2020 the UK National Institute for Health and Care Excellence (NICE) published diagnostic guidance on the use of high-sensitivity troponin tests for the early rule-out of acute myocardial infarction in adults presenting with acute chest pain and suspected NSTEMI.[75]
Overall NICE concluded that it is possible to rule out NSTEMI using a high-sensitivity troponin T or I assay if the levels are below the diagnostic threshold (99th percentile), or a threshold at or near the limit of detection of the assay, on arrival and at 30 minutes to 3 hours later. They also recommended, in selected patients, that a single sample on presentation using a threshold at or near the limit of detection may be used to rule out NSTEMI.
Their advice was based on a systematic review and health economic analysis prepared by an External Assessment Group.[85]
NICE found no strong evidence to differentiate between high-sensitivity troponin tests, and when used in an early rule-out strategy all were cost effective compared with the standard troponin assay. They therefore recommended a range of early rule-out strategies (both single and multiple sample) including the ESC 0 and 1 hour algorithm.
However, they did stipulate that further research is required to explore the optimal testing strategies for these assays in subgroups including sex, age, ethnicity, and renal function.
Chest x-ray
The National Institute for Health and Care Excellence (NICE) recommends ordering a chest x-ray only if you suspect other diagnoses or to rule out complications of ACS.[74] However, in our expert’s opinion you should order a chest x-ray for all patients with acute chest pain to look for other causes, such as pneumothorax or a widened mediastinum in aortic dissection, or complications of ACS such as pulmonary oedema due to heart failure.
Practical tip
Ensure an ECG is recorded and interpreted before considering a chest x-ray. If the ECG indicates STEMI, refer the patient immediately to cardiology for consideration of primary percutaneous coronary intervention to avoid the delay that may be associated with requesting a chest x-ray.
Full blood count
Check full blood count to evaluate:
Thrombocytopenia to estimate risk of bleeding; NSTEMI treatment increases the risk of bleeding
Possible secondary causes of NSTEMI (i.e., secondary blood loss, anaemia).
Urea, electrolytes, and creatinine
Measure renal function to:
Determine serum creatinine and estimated glomerular filtration rate (eGFR); these are key elements in assessing the Global Registry of Acute Coronary Events (GRACE) risk score[5] [ GRACE Score for Acute Coronary Syndrome Prognosis Opens in new window ]
Prevent contrast-induced nephropathy if an invasive strategy is planned in a patient with chronic kidney disease (CKD).[1][5]
Be aware that a patient with CKD may have a chronically raised troponin.[1] Patients with CKD may also have electrolyte abnormalities that can cause ECG abnormalities.
Liver function tests
Measure liver function to include in the assessment of bleeding risk before starting anticoagulation.
Blood glucose
Check blood glucose in any patient with known diabetes or hyperglycaemia on admission to hospital, regardless of a history of diabetes.[5][72]
Monitor blood glucose levels frequently if the patient has known diabetes or hyperglycaemia on admission.[5][72]
Manage hyperglycaemia in patients admitted with confirmed NSTEMI by keeping blood glucose <11 mmol/L (198 mg/dL) while avoiding hypoglycaemia.[72] See Management of hyperglycaemia in Management recommendations.
C-reactive protein (CRP)
NICE does not recommend using CRP to diagnose an ACS.[74] However, in our expert’s opinion, CRP is commonly ordered to rule out other causes of acute chest pain (e.g., pneumonia).
Consider in some patients
Echocardiography
Organise urgent echocardiography for any patient with signs of acute heart failure or haemodynamic instability or who is in cardiac arrest.[5] This should only be performed by those with specialist training.
Use a point-of-care transthoracic echocardiogram to:
Look for regional wall motion abnormalities of the left ventricle in patients with an atypical presentation or equivocal ECG.[1][5][86][87]
Look for mechanical complications of acute MI (see Acute MI complications – mechanical below):[87]
Left ventricular function
Right ventricular function
Ventricular septal rupture
Left ventricular free wall rupture
Acute mitral regurgitation
Pericardial effusion
Cardiac tamponade.
Suggest alternative aetiologies associated with chest pain (e.g., acute aortic disease, pulmonary embolism).[5][87]
A pre-discharge echocardiogram is indicated for all patients post-acute MI to assess left ventricular function after coronary reperfusion therapy and to guide prognostication.[72][88]
Invasive coronary angiography
Get urgent input from a senior colleague or cardiology if the patient is clinically unstable or has any very high-risk features (as outlined below) to arrange immediate invasive coronary angiography (ICA) (with the intent to perform revascularisation by percutaneous coronary intervention [PCI] if indicated).[72] Do not wait for the results of troponin testing.[5] This includes any patient with:[72][73]
Ongoing or recurrent pain despite treatment
Haemodynamic instability (low blood pressure or shock) or cardiogenic shock; see Shock
Recurrent dynamic ECG changes suggestive of ischaemia
Acute left ventricular failure, presumed secondary to ongoing myocardial ischaemia; see Acute heart failure
A life-threatening arrhythmia (ventricular tachycardia or ventricular fibrillation) or cardiac arrest after presentation; see Sustained ventricular tachycardias[5]
Mechanical complications such as new-onset mitral regurgitation.[5]
An inpatient invasive strategy is recommended for most patients presenting with NSTE-ACS; the timing of this is guided by an early risk assessment, which divides patients into very high risk, high risk, or non-high risk.[5] High-risk patients should have ICA within 24 hours.[5] Patients without high-risk features can be managed based on clinical suspicion; conservative management without early angiography may be an option for very low-risk patients.[5][72][89] In older patients (>75 years) studies have shown that an early invasive strategy for NSTE-ACS does not affect the risk of cardiovascular death, but does lead to a significant reduction in subsequent non-fatal MI and need for further revascularisation procedures.[90][91]
Perform further risk assessment after a final diagnosis has been made and treatment administered. For more information, see Risk assessment in Management recommendations.[5]
Evidence: Risk assessment
In people with NSTEMI, risk assessment tools should be used to predict their future risk of adverse cardiovascular outcomes and mortality. European and UK guidelines suggest using the Global Registry of Acute Coronary Events (GRACE) risk score.
In its guidance on ACS (last updated 2020, although evidence not changed from 2013) the UK National Institute of Health and Care Excellence (NICE) evaluated the evidence for methods of patient risk stratification.[72]
NICE included studies where the non-ST-segment elevation ACS population numbered >500 and the study population contained ≥60% people with NSTEMI or unstable angina.
They identified 14 observational studies assessing a total of eight risk scores. Five of these studies compared the performance of two or more different risk scores.
NICE reported discrimination (the ability to accurately distinguish high-risk from low-risk patients, measured with the c-statistic) and calibration (the ability to estimate the actual risk of an adverse outcome).
GRACE versus Platelet glycoprotein IIb/IIIa in Unstable angina: Receptor Suppression Using Integrilin (PURSUIT) risk scores:
Two studies (Canadian ACS-1 [n=2925] and ACS-2 [n=1728] registries) in people with non-ST-segment elevation ACS showed no difference, with both having good discrimination for mortality in-hospital and at 1 year.[92][93]
PURSUIT, however, had poor calibration meaning it consistently overestimated the risks compared with GRACE.[92]
PURSUIT, GRACE, and the Predicting Risk of Death in Cardiac Disease Tool (PREDICT) all seemed to have better discrimination for mortality compared with the Thrombolysis In Myocardial Infarction (TIMI) score.
In the Canadian ACS-2 registry, PURSUIT and GRACE had significantly better discrimination than TIMI for mortality in-hospital and at 1 year (in-hospital mortality: PURSUIT c-statistic 0.80 vs. GRACE c-statistic 0.81 vs. TIMI c-statistic 0.68; 1 year mortality: PURSUIT c-statistic 0.77 vs. GRACE c-statistic 0.79 vs. TIMI c-statistic 0.69).[93]
Based on registry data from the Mayo Clinic in the US (people with confirmed MI, n=717), PREDICT had significantly better discrimination of mortality at 28 days (PREDICT c-statistic 0.78 vs. TIMI c-statistic 0.59, P <0.001 between risk scores).[94]
In the Myocardial Infarction National Audit Project (MINAP) database of people in England and Wales with ACS (n=100,686), complex scores with a greater number of components (PURSUIT and GRACE) were compared with more simple models (Simple Risk Index [SRI] and Evaluation of Methods of Management of Acute Coronary Events [EMMACE]).[95]
All four scores showed similarly high discrimination for predicting mortality in-hospital and at 30 days.
The 2023 European Society of Cardiology (ESC) guidelines for the management of acute coronary syndromes recommend that risk scores should be considered for estimating prognosis.[5]
The ESC specifies that the GRACE risk score is the most accurate for risk stratification
In a retrospective study comparing TIMI and GRACE scores, GRACE performed better in predicting mortality (in-hospital and at 6 months).[96]
GRACE performed better than TIMI in a 2012 meta-analysis that included 18 validation cohorts (n=56,673) of people with NSTEMI (TIMI: c-statistic 0.54 [95% CI 0.52 to 0.57] for short-term studies and 0.67 [95% CI 0.62 to 0.71] for long-term studies; GRACE c-statistic 0.83 [95% CI 0.79 to 9.87] for short-term studies and 0.80 [95% CI 0.74 to 0.89] for long-term studies).[97]
GRACE has also been shown to perform better than subjective physician assessment for predicting mortality or myocardial infarction.[98][99]
Evidence: Use of risk score to guide early invasive strategy
The Global Registry of Acute Coronary Events (GRACE) risk score may be used to identify patients who would benefit from an early invasive strategy.
In its 2020 guidance on ACS, the National Institute for Health and Care Excellence (NICE) identified one study, the Timing of Interventions in Acute Coronary Syndromes (TIMACS) randomised controlled trial, in which the GRACE 6-month mortality model was used to perform a prespecified subgroup analysis of early versus delayed intervention based on patient risk.[72][100]
In patients classified as high risk (GRACE score >140) the primary outcome, a composite of death, myocardial infarction, or stroke at 6 months, occurred less with early intervention (hazard ratio [HR] 0.65, 95% CI 0.48 to 0.89).
For those at low or moderate risk (GRACE score ≤140), there was no significant difference between early and delayed intervention (HR 1.12, 95% CI 0.81 to 1.56).
NICE has a new recommendation in its 2020 guidance to consider coronary angiography within 72 hours of admission for people with unstable angina and NSTEMI who have a predicted 6-month mortality >3.0%.[72][101][102]
The European Society of Cardiology (ESC) 2023 guidelines recommend a GRACE score >140 as a criterion for early invasive treatment (coronary angiography within 24 hours of hospital admission) based on the TIMACS and Very Early veRsus Deferred invasive evaluation using Computerised Tomography (VERDICT) studies.[5][100][103]
However, it is unclear whether using the GRACE risk score improves overall patient care and outcomes.
A cluster-randomised trial, the Australian GRACE Risk score Intervention Study (AGRIS), investigated the impact of the GRACE risk score compared with standard care on inpatient angiography, prescription of guideline-recommended medications, and referral to cardiac rehabilitation.[104] The results showed that the impact of GRACE was limited to inpatient angiography.[105]
Another cluster-randomised trial, the UK GRACE Risk score Intervention Study (UKGRIS), compared use of the GRACE risk score and associated guidelines with standard care and found no difference in adherence to guideline-recommended management, or in time to a subsequent cardiovascular event.[106]
Use of this content is subject to our disclaimer