Details
Anticoagulants are among the most frequently prescribed medications in the US.
The most common indications are:
Stroke prevention in patients with atrial fibrillation
Prevention and treatment of venous thromboembolism (VTE)
Prevention of valve thrombosis and arterial thromboembolism in patients with prosthetic heart valves
Atrial fibrillation affects 700 to 775 per 100,000 people in the US.[1] VTE is a relatively common medical problem with a yearly incidence of approximately 1 in every 1000 adults and a lifetime risk of approximately 8%. It is estimated that more than 1.8 million people in the US will have VTE by the year 2050.[2][3][4] Therefore, the number of patients receiving anticoagulants is substantial and is expected to grow with time.
Management of anticoagulants is challenging as they may cause adverse drug events (ADEs), specifically bleeding, which leads to substantial morbidity and mortality. Anticoagulants are the most common drug class associated with an inpatient adverse event and have been shown to be the cause of 41% of deaths attributed to an ADE.[5] Warfarin is the second most commonly implicated drug in emergency department visits for ADEs in the US.[6] Careful and informed management of anticoagulants is therefore critical.
Anticoagulants inhibit the formation and propagation of blood clots and are used in the treatment of many conditions. While individual anticoagulants are specifically approved for a number of disorders, the use of anticoagulants is off-label for others. The level of evidence for use of anticoagulants varies, with a generally lower level of evidence for more rare disorders.
Anticoagulants can be given either orally or parenterally. Warfarin had been used for more than 50 years before dabigatran, the first alternative (non-vitamin K antagonist) oral anticoagulant, was introduced. Since then, many other alternative oral anticoagulants have become available (e.g., apixaban, edoxaban, rivaroxaban). As these newer agents result in anticoagulation by different mechanisms of action than warfarin, a new nomenclature was needed.[7] The naming of these new drugs has been controversial and has evolved over time. Published terms have included: new, novel, or non-vitamin K antagonist oral anticoagulants (NOACs); target-specific oral anticoagulants (TSOACs); and direct oral anticoagulants (DOACs). A 2014 survey study administered to 150 leaders of 16 professional societies in North America and Europe identified DOACs as the favored term.[8] As new therapeutic agents, which target Factors XI and XII, are emerging, a new nomenclature system has been developed based on route of administration and specific mechanism of action.[8][9]
When deciding to initiate anticoagulation therapy, the risk of bleeding should be weighed against the therapeutic benefits. There are few absolute contraindications; however, anticoagulants should not be used in patients with active bleeding or in patients in whom imminent hemorrhage is anticipated. Before administering anticoagulation therapy, the risk of bleeding, as well as any risks associated with the condition being treated, should be assessed.
Anticoagulants are often initiated in primary care (e.g., for prevention of stroke and arterial thromboembolism in patients with atrial fibrillation, patients with acute venous thromboembolism who do not require hospitalization). Initiating anticoagulation in hospitalized patients follows the same general principles as in the outpatient setting, with the exception that unfractionated heparin and other short-acting intravenous agents are options for these patients.
The initiation of anticoagulant therapy differs according to the agent(s) used, and the indication for therapy. For initial treatment of VTE (“initiation phase”), apixaban and rivaroxaban may be used at a higher dose, after which the treatment dose (“treatment phase”) is used. Initiation of dabigatran and edoxaban requires parenteral anticoagulation first for 5-10 days before electing dabigatran or edoxaban for VTE therapy. Warfarin (and other vitamin K antagonists), when utilized for VTE treatment, must be given with overlapping parenteral anticoagulant therapy, which is then continued until the INR is ≥2.0 for at least 24 hours and at least 5 days of overlap has been achieved. In most patients prescribed an anticoagulant for atrial fibrillation, the treatment dose of the oral agent is started without the need for parenteral therapy or an initiation-dose regimen. In post acute ischemic stroke patients initiated on anticoagulation in the setting of atrial fibrillation, a strategy which includes use of a parenteral anticoagulant for initiation "bridging" may be associated with worse outcomes, so typically should be avoided in favor of starting oral therapy when the risk of hemorrhagic transformation of the stroke has sufficiently abated.[10][11][12]
Anticoagulation therapy requires regular maintenance and re-evaluation. Warfarin requires the most laboratory monitoring of any anticoagulant. It is dosed to achieve a target INR value that differs according to the indication (usually in the range of 2.0-3.5). INR should be performed frequently until a stable dose is achieved, after which it is typically monitored every 4 to 12 weeks depending on clinical circumstances. There is no need for routine laboratory monitoring in patients taking direct oral anticoagulants, though periodic monitoring of renal and hepatic function is suggested.[13][14][15][16]
The need for elective surgery or an invasive procedure in a patient taking anticoagulants is a particularly challenging situation. Key decisions include: determining whether the anticoagulant needs to be interrupted; the timing of interruption; and for warfarin, whether to offer bridging therapy (i.e., use of a shorter-acting parenteral anticoagulant, such as low molecular weight heparin, to reduce the period without effective anticoagulation while warfarin is held). These decisions are based on the bleeding risk associated with the procedure, as well as the patient’s thromboembolic risk when anticoagulants are held. Substantial evidence on the timing of interruption and reinstitution of therapy has emerged, and guidelines inform these decisions.[17][18] Use of “bridging” injections during interruption of oral anticoagulant therapy is infrequently indicated and should be used only in highly selected circumstances.[17][18]
Concomitant antithrombotic agents increase bleeding risk. For patients on warfarin and an antiplatelet agent, more frequent INR monitoring, maintaining an INR of 2.0 to 2.5, and using low-dose aspirin and clopidogrel as the preferred antiplatelet regimen may decrease the risk of bleeding. The greatest bleeding risk is in patients that require dual antiplatelet therapy in conjunction with anticoagulation (also known as triple antithrombotic therapy). There are only a few indications that definitively require dual or triple antithrombotic therapy. Triple antithrombotic therapy should be avoided in most patients and, when used, should be continued for the shortest possible period and only in high thrombotic risk patients.[19] Antiplatelet agents should not be combined with anticoagulants for the purpose of primary prevention of cardiovascular events.
Management of bleeding during anticoagulation requires a comprehensive evaluation and various interventions to mitigate the risk of morbidity and mortality (e.g., source control, blood products, nonspecific prohemostatic agents, and reversal agents). Reversal agents may be general (replacing coagulation proteins) or specific (targeting the antithrombotic drug(s) used by the patients). All reversal agents carry risks which must be weighed against the benefit of reversal on controlling bleeding. After bleeding has been controlled, the patient should be assessed and a decision made whether and, if so, when to resume anticoagulation.
[Figure caption and citation for the preceding image starts]: Perioperative management of vitamin K antagonists (warfarin)Adapted by BMJ Knowledge Centre from Douketis JD et al. Chest. 2022 Nov;162(5):e207-43; used with permission [Citation ends].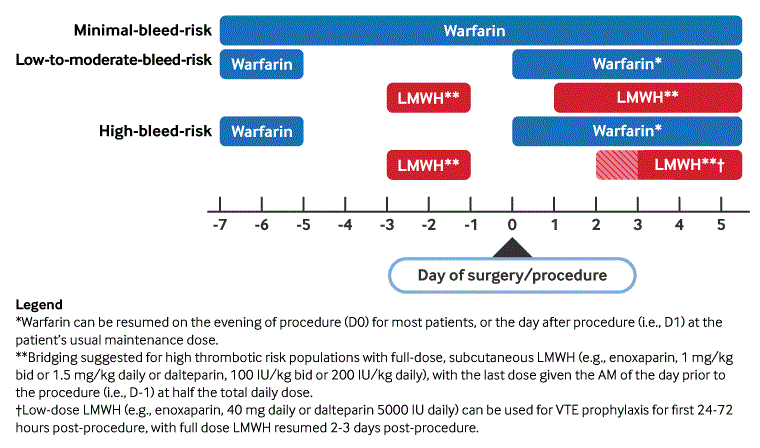
[Figure caption and citation for the preceding image starts]: Perioperative management of direct oral anticoagulants (DOACs)Adapted by BMJ Knowledge Centre from Douketis JD et al. Chest. 2022 Nov;162(5):e207-43; used with permission [Citation ends].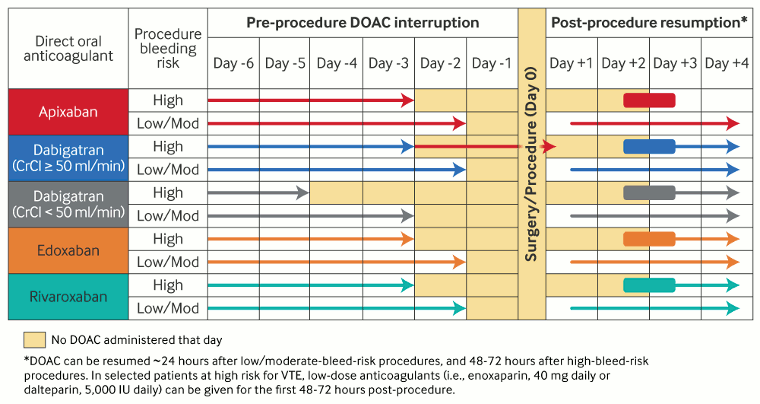
[Figure caption and citation for the preceding image starts]: Approach to the management of acute hemorrhage in an anticoagulated patientProduced by SM Stevens, MD and SC Woller, MD; used with permission [Citation ends].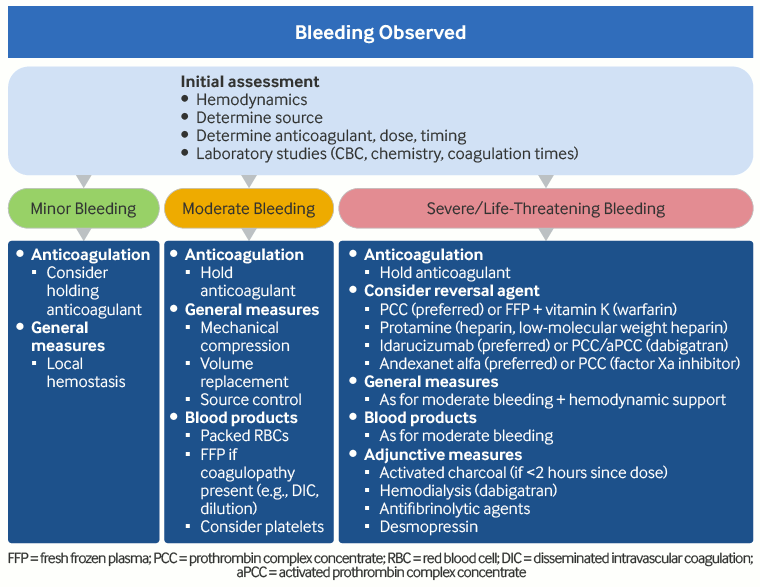 [Figure caption and citation for the preceding image starts]: Risk stratification for procedural bleed risk (* No residual anticoagulant effect at the time of procedure [i.e., four to five drug half-life interruptions pre-procedure]; † Includes spinal and epidural anesthesia or any other neuraxial [e.g., pain management] intervention; consider not only absolute risk for major bleeding but potentially devastating consequences of epidural bleeding and associated lower limb paralysis; ‡ Some residual anticoagulant effect allowed [i.e., two to three drug half-life interruptions pre-procedure]; § Radial approach may be considered minimal bleed risk compared with femoral approach; ¶ Procedure can be safely done under full-dose anticoagulation [may consider holding DOAC dose day of procedure to avoid peak anticoagulant effects])Douketis JD et al. Chest. 2022 Nov;162(5):e207-43; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Risk stratification for procedural bleed risk (* No residual anticoagulant effect at the time of procedure [i.e., four to five drug half-life interruptions pre-procedure]; † Includes spinal and epidural anesthesia or any other neuraxial [e.g., pain management] intervention; consider not only absolute risk for major bleeding but potentially devastating consequences of epidural bleeding and associated lower limb paralysis; ‡ Some residual anticoagulant effect allowed [i.e., two to three drug half-life interruptions pre-procedure]; § Radial approach may be considered minimal bleed risk compared with femoral approach; ¶ Procedure can be safely done under full-dose anticoagulation [may consider holding DOAC dose day of procedure to avoid peak anticoagulant effects])Douketis JD et al. Chest. 2022 Nov;162(5):e207-43; used with permission [Citation ends].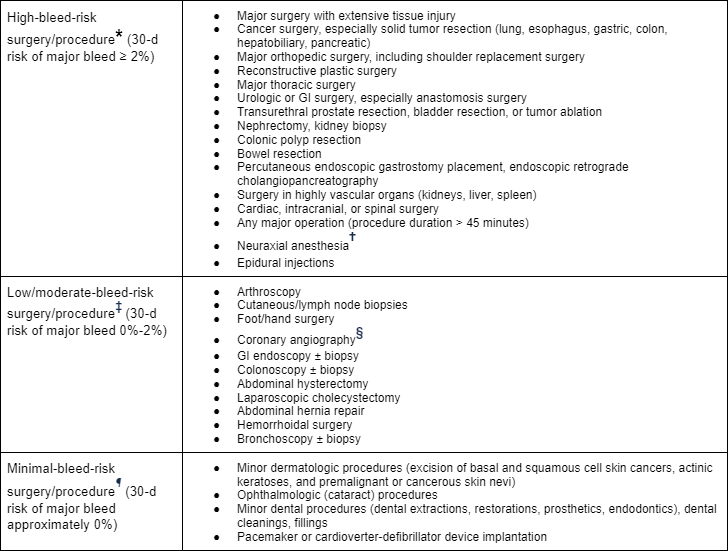
[Figure caption and citation for the preceding image starts]: Summary of advantages and disadvantages of reversal agents (UFH = unfractionated heparin; LMWH = low molecular weight heparin; TRALI = transfusion-related acute lung injury *Guidelines support the use of 4-factor PCC in patients with life-threatening bleeding)Created by the BMJ Knowledge Centre [Citation ends].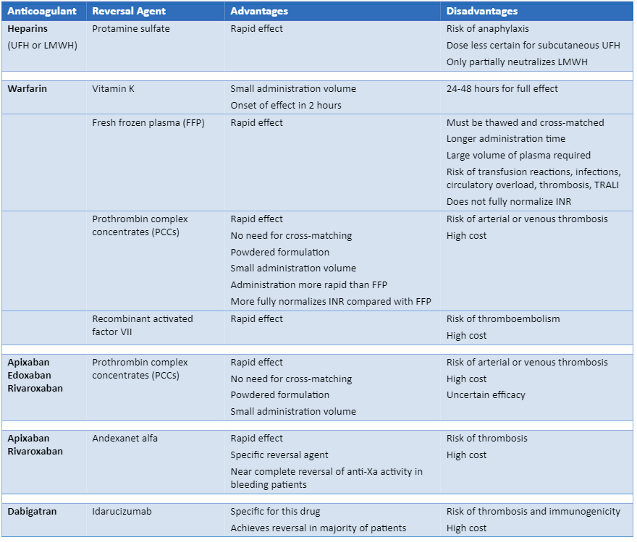
Thrombotic conditions can generally be divided into arterial thromboembolism (ATE) and venous thromboembolism (VTE). Anticoagulants are indicated in both ATE and VTE for the treatment of existing thrombosis and for the primary and secondary prevention of thrombosis.
Treatment of existing thrombosis:
Acute VTE (pulmonary embolism and deep vein thrombosis)
VTE in unusual sites (e.g., splanchnic vein thrombosis, cerebral venous sinus thrombosis) [
 ]
]
Intracardiac and left ventricular (LV) thrombus[20]
Acute coronary syndrome
Acute ATE (usually attributable to cardioembolism) in arterial sites such as the cerebral arteries or the extremities
Heparin-induced thrombocytopenia with acute thrombosis
Thrombosis affecting medical devices (e.g., artificial heart valves, hemodialysis fistulas)
Primary prevention of thrombosis:
Patients with atrial fibrillation with an increased risk for stroke and other ATE
Patients with mechanical heart valves to prevent stroke and ATE [
 ]
]
Hospitalized medical, surgical, or traumatic nonsurgical patients at an elevated risk of hospital-associated VTE, including patients hospitalized with COVID-19[21][22][23]
VTE prophylaxis (post-hospitalization) following: major orthopedic surgeries (i.e., hip and knee replacement surgery, hip fracture surgery); abdominal and pelvic surgery for cancer; moderate-to-severe trauma without surgery; and possibly in selected high-risk patients after medical hospitalization[24][25]
Selected patients with non-ischemic cardiomyopathies at risk for LV thrombus[20]
Ambulatory patients with cancer at intermediate- to high-risk of thrombosis (Khorana score ≥2), receiving systemic therapy[26][27][28][29]
Patients with multiple myeloma receiving thalidomide or lenolidomide-based regimens with chemotherapy and/or dexamethasone[29]
Medical devices (e.g., mechanical circulatory support and extracorporeal membrane oxygenation circuits)
Heparin-induced thrombocytopenia until platelet count recovery.[30]
Secondary prevention of thrombosis:
Patients with a history of VTE for which the risk of recurrent thrombosis is estimated to outweigh the risks and burden of continued anticoagulation therapy
Patients with a history of cardioembolic stroke or other ATE in the setting of atrial fibrillation or antiphospholipid syndrome
Prevention of recurrent miscarriages (presumed to be related to thrombosis impairing placental function, especially in antiphospholipid syndrome); although the efficacy of anticoagulants for this indication is uncertain.[31]
Specific anticoagulants are approved for a variety of indications; complete prescribing information and society guidelines should be consulted before initiating treatment.
The patient population in which anticoagulation is used is heterogeneous, and the rate of outcome events (e.g., stroke in patients with atrial fibrillation) that anticoagulation is intended to prevent varies greatly based on individual patient risk, highlighting the need for individualized risk assessments.
The risk of arterial and venous thrombosis in patients with atrial fibrillation, mechanical heart valves, and venous thromboembolism (VTE) can be estimated.[32][33][34] Formalized risk assessment scores for the outcome of stroke among patients with atrial fibrillation exist (e.g., CHADS2, CHA2DS2-VASc), and based on specific patient characteristics, the annual risk for stroke is estimated to range from 0.2% to >12%.[35][36] The risk of recurrent venous thrombosis after an initial thromboembolic event varies based on the presence, robustness, and perpetuity of thrombosis risk factors at the time of diagnosis; it ranges from a 5-year risk of <3% among patients with surgically-provoked thrombosis, to an annual risk of >15% in patients with thrombosis and cancer.[37][38] In patients with mechanical heart valves, the relative risk for thromboembolism and valve thrombosis among patients taking warfarin compared to no therapy was 0.21 and 0.11, respectively.[33]
Direct oral anticoagulants (DOACs) appear to be associated with a similar or lower risk of major bleeding compared to warfarin when used for the treatment of VTE or the prevention of stroke in patients with atrial fibrillation.[32][39][40][41][42][43][44][45][46][47] This comparative safety may increase the likelihood of anticoagulant use among patients who would otherwise not be considered candidates for anticoagulation and for those patients where the bleeding risk associated with long-term anticoagulation therapy may be identified as acceptable.[45][48][49][50] Emerging evidence, largely observational, is available comparing outcomes directly between different DOACs. Large registries which have utilized stratification by propensity score or other methods to mitigate lack of randomization have suggested similar efficacy between agents, and a tendency toward less bleeding associated with apixaban use.[51][52][53]
An elevated risk of bleeding (see contraindications sections) and thrombosis often coexist.[54][55] In these circumstances, a careful discussion surrounding the risks and uncertainties associated with anticoagulation therapy is warranted. Formalized attempts to measure and qualify a patient’s values and preferences may inform the role of anticoagulation therapy when there is uncertainty surrounding the risks and benefits.[56]
[ Atrial Fibrillation CHA(2)DS(2)-VASc Score for Stroke Risk Opens in new window ]
[ Atrial fibrillation CHADS(2) score for stroke risk Opens in new window ]
There are few absolute contraindications to anticoagulation; however, anticoagulants should not be used in patients with active bleeding or in patients where imminent hemorrhage is anticipated.
Relative contraindications to anticoagulation are commonly encountered though evidence suggests that clinicians overestimate the risk of bleeding and refrain from using anticoagulation therapy in patients who would benefit.[57][58] Therefore, before administering anticoagulation therapy, the risk of bleeding, as well as any risks associated with the condition being treated, should be assessed and balanced. Patients at elevated risk of bleeding who receive anticoagulants should be carefully educated and monitored.[32][59]
Contraindications also exist for specific anticoagulants, and the full prescribing information of a specific drug should be consulted to review these. For example, warfarin is contraindicated in patients with warfarin-induced skin necrosis; unfractionated heparin (UFH) and low molecular weight heparin (LMWH) are contraindicated in patients who have a high probability of (or are diagnosed with) heparin-induced thrombocytopenia; and direct oral anticoagulants (DOACs) may be contraindicated in patients who are taking certain concomitant medications that influence the metabolism of the DOAC or who have specific degrees of renal impairment.[13][14][15][16][60] Additionally, pregnancy is a contraindication for many anticoagulants, including warfarin (for all indications but mechanical heart valves) and the DOACs.
Bleeding risk should be assessed before deciding whether anticoagulation therapy can be initiated. Bleeding risk assessment scores are a useful tool to help achieve this.
In patients with atrial fibrillation who are candidates for anticoagulation for stroke prevention, there are multiple risk factors for bleeding, including advanced age, poorly controlled hypertension, a history of major bleeding, underlying organ dysfunction, historically poor anticoagulation management, and the concomitant ingestion of substances associated with an increased bleeding risk such as alcohol, antiplatelet medications, or nonsteroidal anti-inflammatory drugs (NSAIDs).[61][62][63][64][65][66]
Formalized bleeding risk assessment scores including HEMORR2HAGES, ATRIA, and HAS-BLED have been derived, variably validated, and compared.[62][66][67][68] It is recommended that patients with a higher bleeding risk be monitored carefully, but this higher risk is not a reason to withhold anticoagulation as the net clinical benefit still favors the use of anticoagulants to prevent stroke in most patients.[69]
[ HAS-BLED Bleeding Risk Score Opens in new window ]
HEMORR2HAGES Score for major bleeding risk Opens in new window
ATRIA Bleeding Risk Score Opens in new window
Several bleeding risk scores have been derived in venous thromboembolism (VTE) populations, but routine clinical use should await additional validation.[70][71][72][73][74][75][76][77]
Anticoagulant therapy will often be initiated in primary care, especially for the prevention of stroke and arterial thromboembolism in patients diagnosed with nonvalvular atrial fibrillation, and in patients with acute venous thromboembolism (VTE) who do not require hospitalization.
Prevention of stroke in nonvalvular atrial fibrillation
Anticoagulants are offered to all patients with atrial fibrillation with a greater than minimal predicted risk of stroke (i.e., a score of ≥1 in males or ≥2 in females according to the CHA2DS2-VASc risk assessment method).[69][78][79] [ Atrial Fibrillation CHA(2)DS(2)-VASc Score for Stroke Risk Opens in new window ]
Apixaban, edoxaban, rivaroxaban, dabigatran, and warfarin are recommended, with the direct oral anticoagulants (DOACs) preferred to warfarin in most patients. Initiation of anticoagulation with loading doses or initial parenteral anticoagulation are not required for this indication in the absence of known acute intracardiac thrombus.[10][11] In patients with renal impairment, a dose adjustment may be required, or warfarin may be preferred.
Acute VTE
For acute VTE, initial therapy (“initiation phase”) differs from subsequent therapy for treatment of the thrombotic event (“treatment phase”). Three different approaches to initiating anticoagulant therapy are used in patients with acute VTE (not requiring hospitalization), depending on the agent selected:
Loading: a higher dose of an oral agent is given initially, followed by a lower dose thereafter (e.g., apixaban, rivaroxaban).
Switching: a parenteral anticoagulant is given initially, then the patient is switched to an oral anticoagulant (e.g., dabigatran and edoxaban should only be initiated after 5-10 days’ treatment with a parenteral anticoagulant and are started when the next dose of the parenteral anticoagulant is due).
Overlapping: a parenteral anticoagulant and an oral anticoagulant are initially given simultaneously, and the parenteral anticoagulant is then discontinued once the oral agent has reached therapeutic levels (e.g., warfarin should be overlapped with a therapeutic dose of a parenteral anticoagulant for a minimum of 5 days and until the INR is >2.0 for at least 24 hours).[80]
Parenteral anticoagulants that can be used in switching and overlapping regimens in outpatients include low molecular weight heparins (enoxaparin, dalteparin), fondaparinux, and more rarely, unfractionated heparin, all administered subcutaneously.[81] The dose of these agents depends on the weight of the patient.
When VTE is diagnosed in the setting of active cancer (cancer-associated thrombosis [CAT]), two options exist. The preferred option is use of an oral factor Xa inhibitor (apixaban, edoxaban, or rivaroxaban) at the same dose used for VTE in patients without cancer. An alternative option is extended use of parenteral anticoagulation with a low molecular weight heparin (LMWH), without transition to an oral agent.[27][32][82][83]
In patients with luminal gastrointestinal cancer, apixaban or LMWH may be the preferred options due to a lower risk of major gastrointestinal bleeding.[32] When extended-duration LMWH is chosen in patients with CAT, dalteparin (loading regimen) is recommended, while use of enoxaparin for this indication is off-label.[84][85][86]
With the advent of DOACs, primary care physicians may also need to transition patients from warfarin:
Warfarin to dabigatran or apixaban: discontinue warfarin and start apixaban or dabigatran when the INR is <2.0.[15][16]
Warfarin to rivaroxaban: discontinue warfarin and start rivaroxaban when the INR is <3.0.[13]
Warfarin to edoxaban: discontinue warfarin and start edoxaban when the INR is ≤2.5.[14]
When transitioning a patient from a DOAC to warfarin, frequent testing and a suitable dosing protocol is required because DOACs may influence the INR.[87] The INR should be checked just prior to the next dose of the DOAC to reduce the influence on the INR.
Initiating anticoagulation in hospitalized patients follows the same general principles as initiating anticoagulation in the outpatient setting. The dose of oral anticoagulants for most indications is the same as the dose in primary care.
Intravenous unfractionated heparin (UFH) is often used for initial anticoagulation in hospitalized patients, as: it does not depend significantly on renal function for elimination; has a short half-life and is readily reversed if bleeding occurs; and is the agent most studied for use in conjunction with thrombolytics. UFH is also often used in the inpatient setting for patients with acute coronary syndrome. However, UFH is likely to have lower efficacy than other anticoagulants, and so should only be chosen for a compelling indication (such as possible use of thrombolytic therapy, extremes of weight, severe renal insufficiency).[88]
The dose of UFH required to achieve a therapeutic effect differs among individuals, and the required dose may even change within the same individual. Frequent measurement of the activated partial thromboplastin time (aPTT) is used to monitor and titrate therapy. Target aPTT values must be determined by each laboratory as they differ according to the instrument and reagents used (the local laboratory determines these by calibration against anti-Xa activity or protamine reversal studies). The aPTT should be obtained 6 hours after the initiation of UFH. If outside of the target range, the dose should be adjusted and the aPTT repeated 6 hours after every dose adjustment. Once three successive aPTT values are in the target range, monitoring can be reduced to once daily.
The dose of UFH and target aPTT depend on the indication:[80]
Acute venous thromboembolism (VTE): initiated with an intravenous bolus dose, which is dependent on body weight, followed by an intravenous infusion. Outcomes are improved when the aPTT reaches the target range (i.e., 1.5 to 2.5 times laboratory control) in 24 hours or less following initiation of therapy.
Acute coronary syndrome: initial dose and target aPTT are lower in these patients (i.e., aPTT should be 1.5 to 2.0 times laboratory control), as they are often at a higher risk of bleeding due to concomitant use of antithrombotic medications, though are still weight-based.
VTE prophylaxis: weight-based doses are not necessary for this indication; rather, a fixed dose of UFH is usually administered subcutaneously every 8 to 12 hours and aPTT is not routinely monitored.
In selected patients, the aPTT may be confounded and fail to reflect the serum heparin level. In these instances, titration through calibrated anti-Xa activity assays is preferred.[89] As calibrated anti-Xa activity correlates more closely to serum heparin levels, and has fewer confounding factors than aPTT, some institutions, especially pediatric facilities, opt for use of anti-Xa assays in all patients. Research has also shown no observable differences in bleeding or thrombosis outcomes when comparing anti-Xa assays to aPTT for UFH monitoring.[90] However, anti-Xa activity assays are more expensive and turnaround time may be longer. Individual institutions generally determine which method is chosen routinely for UFH management.
Less specific dosing information is available when heparin is used for other indications such as post-acute cardioembolic stroke or in the periprocedural period of cardiac or vascular surgeries. The same dosing strategy used for acute coronary syndrome is often employed in these settings.
Low molecular weight heparin (LMWH) may also be used in this setting for the treatment of acute venous thromboembolism (VTE), prevention of VTE, and acute coronary syndrome (ACS) in hospitalized patients. These agents (e.g., enoxaparin, dalteparin) are administered subcutaneously, although an intravenous bolus may be given initially in selected patients with ACS. The dose is based on weight, differs depending on the indication, and requires renal dose adjustment for low creatinine clearance.[80] Monitoring with anti-Xa activity is rarely required. Fondaparinux may also be used for the treatment and prevention of VTE. The dose depends on the patient’s weight and is administered subcutaneously. Caution should be used in patients with renal impairment for both classes of parenteral anticoagulants.
Patients receiving parenteral anticoagulants in the hospital, and who require ongoing anticoagulation following discharge (e.g., patients with venous thromboembolism [VTE]), are transitioned from parenteral to oral anticoagulants, except in select instances when ongoing parenteral anticoagulation with low molecular weight heparin (LMWH) is preferred (e.g., pregnancy, patients with cancer-associated thrombosis [CAT] who are not candidates for an oral Xa inhibitor). The method of transition depends on the indication for anticoagulant therapy, as well as the oral anticoagulant chosen.
For patients receiving therapy for acute VTE who are being transitioned to warfarin, parenteral anticoagulation and oral warfarin must be overlapped for a minimum of 5 days, and the parenteral anticoagulant must not be stopped until the INR is ≥2.0 for at least 24 hours.
For VTE patients transitioning to oral dabigatran or edoxaban, parenteral anticoagulation must be given for 5-10 days first, after which the patient switches to the oral agent.
For VTE patients receiving a parenteral anticoagulant and who will then transition to apixaban or rivaroxaban, it is reasonable to give the loading dose followed by the approved dose recommendations once the decision is made to change to oral therapy. No overlap is required.[91]
When VTE is diagnosed in the setting of active cancer (CAT), two options exist. The preferred option is use of an oral factor Xa inhibitor (apixaban, edoxaban, or rivaroxaban) at the same dose used for VTE in patients without cancer. An alternative option is extended use of parenteral anticoagulation with a LMWH, without transition to an oral agent.[32][82] In patients with luminal gastrointestinal cancer, apixaban or LMWH may be the preferred options due to a lower risk of major gastrointestinal bleeding.[32] When extended-duration LMWH is chosen in patients with CAT, dalteparin (loading regimen) is recommended, while use of enoxaparin for this indication is off-label.[84][85][86]
In patients with arterial thromboembolism or atrial fibrillation receiving parenteral anticoagulants, the transition to oral anticoagulation is more controversial. However, it is reasonable to use the same standards as VTE patients in those with an acute thrombosis.
Heparin-induced thrombocytopenia (HIT) requires inpatient management and unique parenteral antithrombotic therapy. After diagnosis, many of these patients will receive initial therapy with an intravenous thrombin inhibitor (argatroban is currently the only approved drug for this indication in the US, while bivalirudin is approved for use in the setting of cardiac procedures in patients with a previous history of HIT).[92][93] Fondaparinux has also been used in patients with normal renal function and HIT; however, it is not approved for this indication.[94] Data for the use of direct oral anticoagulants (DOACs) is very limited; they are not approved for this indication although one guideline has endorsed their use.[30] If DOACs are used in the setting of HIT complicated by thrombosis, it is reasonable to use the VTE treatment dosing strategy, including the initiation phase.[30] Evidence does not exist to inform the timing of DOAC initiation however it is reasonable to initiate a DOAC once platelet counts are recovered.
Argatroban: as with heparin, dose titration, dose adjustment, and monitoring are required. The drug is given as an intravenous infusion with no initial bolus dose required. Since its initial approval, additional studies have been published regarding its use and current guidelines suggest a dosing strategy that differs from the approved dose.[95] The initial dose should be lowered in patients with heart failure, multiple organ failure, anasarca, or post-cardiac surgery. Activated partial thromboplastin time (aPTT) is the preferred test with a target value of 1.5 to 3 times the patient’s baseline value. The aPTT should be obtained 2 hours after the initial dose. If outside of the target range, the dose should be adjusted and the aPTT repeated 2 hours after every dose adjustment. Once three successive aPTT values are in the target range, monitoring can be reduced to once daily.
Bivalirudin: approved for use in the periprocedural period following percutaneous coronary intervention in patients with a history of HIT (replacing heparin). It is given as an intravenous bolus followed by an intravenous infusion which may be continued for up to 20 hours after the procedure. A dose reduction is required in renal impairment. Dose titration and monitoring are achieved by using the activated clotting time (ACT) or aPTT. ACT should be obtained 5 minutes after the initial bolus dose to verify that the dose is adequate; however, the frequency of repeat testing is not well defined. The target ACT is >300 seconds. The target aPTT is 1.5 to 2.5 times the patient’s baseline value or mean of normal laboratory range. The aPTT should be obtained 2 hours after the initial dose. If outside of the target range, the dose should be adjusted and the aPTT repeated 2 hours after every dose adjustment. Once three successive aPTT values are in the target range, monitoring can be reduced to once daily. Bivalirudin may also be used for the treatment of HIT; however, this indication is not approved. For this indication, no initial bolus is required.[95]
Warfarin therapy is challenging in patients on argatroban as the latter prolongs the INR (although this effect usually diminishes 3-4 hours after argatroban is stopped). Warfarin may be started when the platelet count has stabilized in the normal range (usually defined as >150,000/mL).[95] Argatroban should be overlapped with warfarin for at least 5 days and should be discontinued only when the INR (based on warfarin alone) has been at least 2.0 for 24 hours. One specific strategy that has been suggested is: administer the two drugs together until the INR is ≥4.0, discontinue argatroban and obtain INR 4 hours later, then resume argatroban at the preceding dose once the specimen has been obtained. If the INR is ≥2.0 on two successive days using this monitoring strategy, discontinue argatroban and continue warfarin alone. If the INR is <2.0, adjust warfarin dose upward and repeat the same testing strategy until two INR values of ≥2.0 have been obtained while argatroban is held. Similar challenges may present when bridging to warfarin with bivalirudin and a strategy of targeting a slightly higher initial INR may be warranted.[96] Use of DOAC rather than warfarin for ongoing anticoagulation following platelet recovery averts this challenge, and is endorsed by guidelines.[30]
Three strategies can be used to select the initial dose of warfarin:
[  ]
]
Clinical algorithm: calculates the estimated stable and starting dose based on several patient characteristics.[97] Clinical variables that predict a higher warfarin dose include: age <55 years; male sex; African-American ethnicity; vitamin K intake >400 micrograms/day; and patient body weight >91 kg.[98] Older age and the presence of comorbidities predict a lower dose requirement.[99] Ethnicity may also influence dose (e.g., mean weekly total dose was 24 mg for Asian-Americans, 31 mg for Hispanics, 36 mg for Caucasians, and 43 mg for African-Americans in one study).[100]
Genetic algorithm: calculates the estimated stable and starting dose based on the results of genetic tests such as CYP450-2C9 genotype and VKOR-C1 haplotype, as well as clinical variables. While the prescribing information for warfarin suggests dose adjustment based on genetic information, randomized trials have found conflicting information regarding the value added by genetic testing. A meta-analysis of 20 randomized trials that enrolled over 5000 patients noted improved time in therapeutic range, and fewer bleeding events, when warfarin was initiated using a genetic algorithm.[101] However, there was no difference in other outcomes, including mortality. Guidelines do not support the use of genetic information for dosing.[102][103][104][105][106][107]
Fixed dose: initiation nomograms based on starting doses of 5 mg/day or 10 mg/day resulted in similar outcomes, with the 5 mg/day starting dose performing better (i.e., more rapid achievement of INR) in older, frail inpatients, and the 10 mg/day dose performing better in younger, healthier outpatients.[108][109] While a fixed-dose strategy has the advantage of simplicity, clinical algorithms result in fewer dose adjustments and out-of-range INR values.
A baseline INR should be obtained prior to initiating warfarin to evaluate for any underlying coagulopathies that might justify a lower warfarin starting dose. It is recommended that the INR be monitored daily for inpatients and every few days for outpatients until a therapeutic INR and stable warfarin dose is reached.[103]
An online tool is available to assist with warfarin initiation dosing, which utilizes clinical variables with or without the addition of genetic information.
Guidelines recommend that all patients with new venous thromboembolism (VTE) remain on anticoagulant therapy for a period of at least 3 months, known as the treatment phase, unless strongly contraindicated (e.g., actively bleeding, very high-risk lesion for bleeding). The goals of this phase are to fully stabilize the original thrombus so that it is no longer prone to propagation or embolization, and to allow onset, and in some cases completion, of recovery from the original event.[32] Anticoagulants do not have significant thrombolytic properties, and dissolution of the original thrombus via the intrinsic fibrinolytic system can take longer than 3 to 6 months.[110]
Upon completion of the treatment phase, the patient should be reassessed to determine whether anticoagulant therapy should be stopped or extended (i.e., therapy with no planned stop date), with the goal of preventing new VTE events. Determining whether to enter into an extended phase of therapy is very challenging; the decision ultimately comes from estimating the risk of a recurrent event without anticoagulation against the risk of major bleeding with therapy, cost, and the inconveniences of continuing therapy. While this decision should always be personalized and informed by the values and preferences of the patient, guidelines provide recommendations to assist in this decision-making process. See Deep Vein Thrombosis and Pulmonary Embolism.
Type of VTE episode
VTE following major transient provocation (e.g., surgery): anticoagulation should be stopped at the conclusion of the treatment phase.
VTE following nonsurgical transient risk factor (e.g., estrogen therapy): anticoagulation should be stopped at the conclusion of the treatment phase in patients with high bleeding risk; suggest that anticoagulation be stopped at the conclusion of the treatment phase in patients with low-to-moderate bleeding risk, although some guidelines suggest anticoagulation be continued for secondary prevention in this group.[32][111]
VTE without apparent precipitating factors (unprovoked VTE): anticoagulation should be stopped at the conclusion of the treatment phase in patients with high bleeding risk; suggest that anticoagulation be continued into the extended phase in patients with low-to-moderate bleeding risk.
Recurrent VTE without apparent precipitating factors (unprovoked VTE): anticoagulation should be continued into the extended phase (no planned stop date) in patients with low-to-moderate bleeding risk. Anticoagulation should be stopped at the conclusion of the treatment phase in patients with high bleeding risk.
VTE in the setting of persistent (non-transient) risk factor: anticoagulation should be continued into the extended phase (no planned stop date) in patients with low-to-moderate bleeding risk. Anticoagulation should be stopped at the conclusion of the treatment phase in patients with high bleeding risk.
VTE in the setting of cancer: anticoagulation should be continued into the extended phase (no planned stop date; until cancer is no longer active) in patients with low-to-moderate bleeding risk; consider continuing anticoagulation into the extended phase in patients with high bleeding risk.[27][32][83]
There are important caveats and limitations to the decision-making process. The classification of the bleeding risk is imprecise. Several bleeding risk scores have been derived in VTE populations, and professional societies have provided guidance, but routine clinical use should await additional validation.[70][71][72][73][74][75][76][77][112] Risk prediction models derived in atrial fibrillation patients have uncertain performance in the VTE population. Guidance statements do not address all possible clinical scenarios (such as recurrent episodes of provoked VTE), and the data supporting these statements were derived from studies covering cases of lower extremity deep vein thrombosis (DVT) and pulmonary embolism (PE), and so may not apply to VTE in less common sites.
Extended phase anticoagulation implies no specific stop date, but trials assessing the benefit of this practice have generally followed patients for only 2 to 4 years. The balance of risks and benefits of anticoagulation beyond this timeframe is uncertain.[32]
While unprovoked VTE carries a high risk for recurrence, not all patients will have a new event if anticoagulation is stopped. Several strategies have been explored to identify subgroups of unprovoked VTE patients who can safely stop anticoagulation. One of these, the HER-DOO2 Risk Prediction score, has undergone both internal and external validation, and has been studied prospectively.[113] However, D-dimer is a component of this score and it is unclear if the same performance occurs with different D-dimer assays.[114] As of yet, none of the other scoring systems are widely validated or recommended by current guidelines.[115][116][117] Likewise, laboratory tests (such as D-dimer), and residual thrombosis on ultrasound have been studied but have limited ability to identify patients who are at low risk for recurrent thrombosis.[116][118]
When selecting patients for possible extended therapy, several factors are important to consider:
The decision is not permanent and should be re-evaluated regularly (i.e., at least annually and with major changes in patient health).[32]
The case-fatality rate of recurrent VTE is less than that of a major bleeding episode and this is also true in patients with cancer.[119][120]
The results of hereditary thrombophilia testing are controversial. With the exception of specific clinical scenarios, results should not be used to determine whether to offer or withhold extended anticoagulation as they are not adequately predictive.[121][122][123]
Patient values and preferences must always be a strong component of this decision.[32]
See Deep vein thrombosis and Pulmonary embolism.
For stroke prevention in atrial fibrillation, as with venous thromboembolism (VTE), the decision to offer anticoagulation includes an assessment of the stroke risk in the absence of anticoagulation versus the risk of bleeding with therapy.
Risk prediction systems have been developed and externally validated for both of these risks; however, stroke and bleeding share many common risk factors.[78][124] The risk of stroke increases with age to a more substantial degree than the risk of bleeding, and the net clinical benefit of anticoagulation increases as the patient ages. Therefore, once it has been decided to start therapy, it is generally with the intent of continuing it indefinitely.
Regular re-evaluation is recommended as a bleeding event or patient preference may influence this decision. An increased predicted bleeding risk (using a prediction model) should be addressed with close monitoring and follow-up.[69][125]
Neither ablation procedures nor medical treatment (e.g., rate or rhythm control) of atrial fibrillation alter the decision to offer and continue anticoagulant therapy.[69] Left atrial appendage closure procedures, either with a percutaneous device or with surgery, offer a means to discontinue anticoagulant therapy in many patients; though a period of antithrombotic therapy of several weeks is required following the closure procedure.[126]
After initiating treatment, anticoagulation therapy requires regular maintenance and re-evaluation. Some agents, most notably warfarin, require regular laboratory monitoring to ensure therapeutic levels are maintained during the treatment period.
Five major principles have been proposed to assure high-quality ongoing management of anticoagulation:[127]
Verify that the patient is taking the medication correctly. For example, regularly ensure that the patient understands the purpose of the medication, the proper dose and timing of the dose, whether the medication needs to be taken with or without food, and that the drug remains affordable to them.
Ensure that any interruptions to therapy are managed appropriately. Determining whether and when to resume anticoagulant therapy following a significant bleeding episode is challenging.
Review and manage any concomitant medications to reduce the risk of important drug interactions and the bleeding risk.
Ensure that the dose is prescribed correctly and is modified for clinical changes (e.g., changes in renal function).
In older patients, optimize blood pressure control to reduce the risks of an intracranial bleed from hypertension or falls from hypotension.
It is also important to review the patient’s values and willingness to continue therapy.[128]
The type and intensity of laboratory monitoring varies widely among the different available anticoagulant agents. However, monitoring does not refer only to laboratory testing but to reviewing the clinical status of the patient and monitoring for circumstances that may impact anticoagulant management. There is no definitive schedule for the frequency of clinical visits after diagnosis and treatment initiation of anticoagulants. Patients with a good understanding of the disease and its management may only require annual visits. Patients at higher bleeding risk or with comorbidities may benefit from more frequent follow-up. Evaluation may include episodes of bleeding, hospitalization, planned invasive procedures, and medication adherence, as well as changes in health status, concomitant medications, diet, activity level, and other factors (e.g., the practicalities of regular monitoring from the patient’s point of view). Monitoring of the patient’s clinical status is advocated regardless of the anticoagulant being used.[127] Reminder systems are associated with enhanced adherence.[129]
Warfarin requires the most laboratory monitoring of any oral anticoagulant. Response to therapy is monitored using the prothrombin time, which is then mathematically transformed into the international normalized ratio (INR), so that values performed at different laboratories produce comparable results.[130] The quality of warfarin management is most commonly measured by calculating the time in the therapeutic range (TTR) expressed as a percentage, with higher values correlated to better patient outcomes.[130][131]
Warfarin is dosed to achieve a target INR value which differs according to the indication:
Standard range (INR target 2.5, range 2.0 to 3.0): most indications.[130]
High range (INR target 3.0, range 2.5 to 3.5): stroke prevention in patients with mechanical mitral valves; or patients with mechanical aortic valves and additional risk factors.[132]
Frequency of testing
INR should be performed frequently until a stable dose is achieved. This is defined as two successive INR values in the target range separated by at least 7 days with no change in dose.[104] Frequency of initial testing ranges from daily (e.g., inpatients) to two to three times per week (e.g., outpatients).[108][109] Frequency should decrease as a stable dose is approached.
Once a stable dose has been achieved, the INR is typically monitored every 4 weeks.[133] When the INR has been stable for a long time (e.g., all INR values within the target range for at least 6-12 months), it can be measured at longer intervals (e.g., every 12 weeks).[130]
Frequency of testing should increase when the dose is altered or the INR goes out of range. A recall interval of 7 days resulted in better outcomes compared to longer intervals after an out-of-range value.[134] One guideline recommends refraining from adjusting the dose when a single INR value is minimally out of range (i.e., ≤0.5 below or above the target value); however, persistent mildly out-of-range values should prompt a dose adjustment.[135] Another study achieved a longer time in the therapeutic range with a fixed-dose adjustment for mildly out-of-range values without an apparent cause. The weekly warfarin dose was increased by 15% if the INR was 1.51 to 1.99, and decreased by 10% if the INR value was 3.01 to 3.99.[136]
Frequency of testing should also increase when a drug known to interact with warfarin is started, discontinued, or the dose of that drug is changed.
Several factors are associated with unstable INR control, including: hepatic dysfunction; changes in thyroid function; fever; cigarette smoking; chewing tobacco; heart failure exacerbations; presence of end-stage renal disease; acute illness; hospitalization; chemotherapy; and prolonged vomiting or diarrhea. These circumstances may warrant more frequent monitoring.[103] A clinical prediction score (SAMe-TT2R2 score) based on patient sex, age, medical history, treatment strategy, tobacco use, and race, has been developed to identify these patients at initiation. Prediction of poor INR control may favor the choice of a direct oral anticoagulant (if not contraindicated) over warfarin.[137] SAMe-TT2R2 score Opens in new window Nonadherence to warfarin is also a major factor that affects INR stability.[138]
Venues for monitoring
Clinics: warfarin is often managed in specialized clinics. Guidelines suggest that these are preferred over standard medical practices, though such services may not be available to all patients.[139] When managed in a standard clinic setting, it is important that a systematic method of management, patient tracking, and reminder systems are in place.[130]
Patient self-testing: patients may perform INR testing without a medical visit, using point-of-care devices. A medical provider is contacted with the result, and the provider communicates any dose instructions and recall interval to the patient. Outcomes are at least as good as those achieved in dedicated anticoagulation clinics, and patient satisfaction is high.[103][140][141][142] [
 ]
INR testing is generally performed weekly in the self-testing environment.
]
INR testing is generally performed weekly in the self-testing environment.
Patient self-management: in addition to testing with a point-of-care device, patients may also be taught to perform their own dose adjustments. While outcomes have been favorable in studies, patients were highly selected and extensively trained with a significant percentage of patients unable to meet self-management qualifications.[142][143][144] This approach is best reserved for highly selected patients, and for systems experienced in working with such patients.
The Anticoagulation Forum Centers of Excellence are a professional society that offer resources to clinics to assist with managing warfarin therapy and recognizes those that have achieved a high set of standards.
Anticoagulation Forum Centers of Excellence Opens in new window
Unfractionated heparin (UFH)
Frequent measurement of the activated partial thromboplastin time (aPTT) is used to monitor and titrate dose. Target aPTT values must be determined by each laboratory as they differ according to the instrument and reagents used. The aPTT should be obtained 6 hours after the initial dose. If outside of the target range, the dose should be adjusted and the aPTT repeated 6 hours after every dose adjustment. Once three successive aPTT values are in the target range, monitoring can be reduced to once daily. Target aPTT depends on indication: 1.5 to 2.5 times laboratory control in acute venous thromboembolism (VTE), and 1.5 to 2.0 times laboratory control in acute coronary syndrome.[80] In selected patients, the aPTT may be confounded and fail to reflect the serum heparin level. In these instances, titration through calibrated anti-Xa activity assays is preferred.[89] Research has shown no observable differences in bleeding or thrombosis outcomes when comparing anti-Xa assays to aPTT for UFH monitoring.[90] Some institutions, especially pediatric facilities, opt for use of anti-Xa assays in all patients. Platelet counts should be monitored regularly.
Low molecular weight heparin (LMWH)
Quantitative monitoring of anti-Xa activity can be performed, but is seldom indicated, and has not consistently been associated with clinically relevant outcomes.[80] Renal function should be monitored periodically and platelet counts should be monitored regularly.
Fondaparinux
Quantitative monitoring of fondaparinux-specific anti-Xa activity can be performed, but is seldom indicated.[80] Renal function should be monitored periodically.
Bivalirudin
Dose titration and monitoring are achieved using the activated clotting time (ACT) or aPTT. The ACT should be obtained 5 minutes after the initial bolus dose to verify that the dose is adequate; however, the frequency of repeat testing is not well defined. Target ACT is >300 seconds. Target aPTT is 1.5 to 2.5 times the patient’s baseline value or mean of normal laboratory range. The aPTT should be obtained 2 hours after the initial dose. If outside of the target range, the dose should be adjusted and the aPTT repeated 2 hours after every dose adjustment. Once three successive aPTT values are in the target range, monitoring can be reduced to once daily.
Argatroban
The aPTT is the preferred test with a target of 1.5 to 3 times the patient’s baseline value. aPTT should be obtained 2 hours after the initial dose. If outside of the target range, the dose should be adjusted and the aPTT repeated 2 hours after every dose adjustment. Once three successive aPTT values are in the target range, monitoring can be reduced to once daily.
There is no need for laboratory monitoring of the anticoagulant effect in patients taking direct acting anticoagulants (DOACs) due to the predictability of their effect. However, there may be clinical circumstances where determining the anticoagulant level of a DOAC is desirable (e.g., major bleeding, need for an urgent invasive procedure, new thrombosis despite treatment, or suspected overdose or nonadherence). Monitoring may be helpful when co-administration of an interacting drug is being considered.[87][145] Finally, there is little evidence for the safety and effectiveness of DOACs at very low body weight (i.e., <50 kg), and monitoring may be helpful in these patients.[87]
A therapeutic level for DOACs is not supported by evidence, as drug levels have not been correlated directly to important patient outcomes. However, the term "on-therapy" has been proposed, with above on-therapy and below on-therapy reflecting very high and very low drug levels, respectively.[146] Standard coagulation time tests have substantial limitations with DOACs and, at best, can only provide a qualitative assessment of DOAC levels depending on the agent, test, and circumstances. This qualitative information may be sufficient for the clinical need in some instances, for example, excluding on-therapy and above on-therapy levels of a drug in a patient needing an urgent procedure.[87][146]
[Figure caption and citation for the preceding image starts]: Qualitative laboratory measurement of direct oral anticoagulants with standard testsProduced by SM Stevens, MD and SC Woller, MD; used with permission [Citation ends].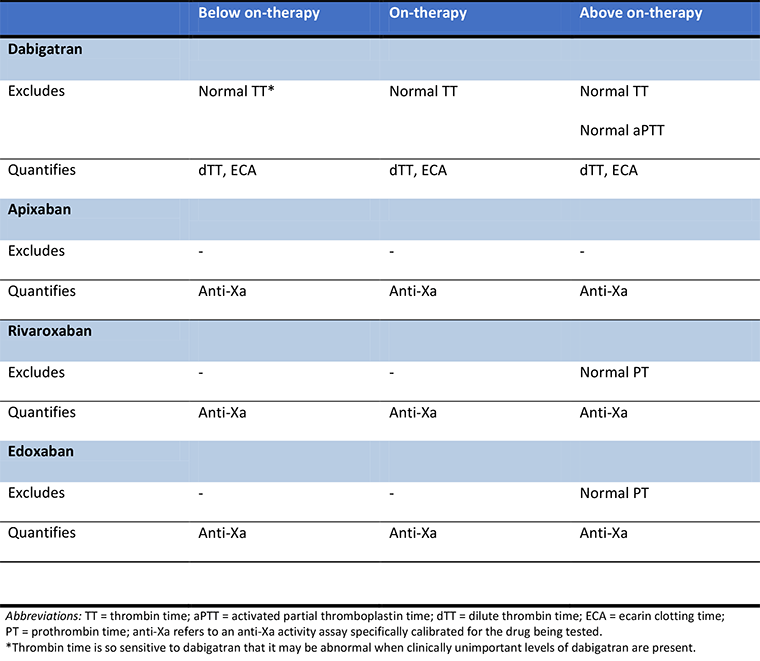
Quantitative testing requires the use of specialized testing such as calibrated anti-Xa activity for oral Xa inhibitors, and ecarin clotting time or dilute thrombin time for dabigatran. These tests are not widely available and results may not be available in a timely manner.[146]
Renal function should be monitored annually in patients with normal renal function and more frequently in patients with renal impairment. DOACs are contraindicated at different degrees of hepatic impairment as determined by the Child-Pugh classification.[13][14][15][16] Patients with known or suspected hepatic disorders should undergo periodic liver function testing.
The need for elective surgery or an invasive procedure in a patient taking anticoagulants is a particularly challenging situation. The primary approach to management is to select the agent, dose, and timing that results in the lowest risk of both bleeding and recurrent thrombosis. Key decisions include: determining whether the anticoagulant needs to be interrupted; the timing of interruption; and for warfarin, whether to offer bridging therapy (i.e., use of a shorter-acting parenteral anticoagulant, such as low molecular weight heparin, to reduce the period without effective anticoagulation while warfarin is held). These decisions are based on the bleeding risk associated with the procedure, which can be classified as high, low/moderate, or minimal, and for warfarin, the underlying risk of thrombosis. Substantial evidence on the timing of interruption and reinstitution of therapy has emerged, and guidelines inform these decisions.[17][18][87]
[Figure caption and citation for the preceding image starts]: Risk stratification for procedural bleed risk (* No residual anticoagulant effect at the time of procedure [i.e., four to five drug half-life interruptions pre-procedure]; † Includes spinal and epidural anesthesia or any other neuraxial [e.g., pain management] intervention; consider not only absolute risk for major bleeding but potentially devastating consequences of epidural bleeding and associated lower limb paralysis; ‡ Some residual anticoagulant effect allowed [i.e., two to three drug half-life interruptions pre-procedure]; § Radial approach may be considered minimal bleed risk compared with femoral approach; ¶ Procedure can be safely done under full-dose anticoagulation [may consider holding DOAC dose day of procedure to avoid peak anticoagulant effects])Douketis JD et al. Chest. 2022 Nov;162(5):e207-43; used with permission [Citation ends].
Certain procedures, such as selected ophthalmologic, dental, and dermatologic procedures, are thought to carry such a low risk of bleeding that anticoagulation need not be interrupted at all.[18][147] Better outcomes have been reported when warfarin is not interrupted for pacemaker placement or catheter ablation for atrial fibrillation.[148][149]
Due to its pharmacokinetics, interrupting warfarin therapy means that there is a period of several days where the patient will not receive sufficient anticoagulation. However, high-quality data have revealed an increase in the risk of bleeding when bridging therapy with parenteral anticoagulants is used, with no reduction in the risk of thrombosis.[150][151][152][153] Newer guidelines and guidance statements suggest that bridging should be offered to fewer patients than suggested in previous recommendations.[18][107][147]
Given the shorter half-lives of direct oral anticoagulants (DOACs) compared to warfarin, bridging therapy is not recommended.[18][87][147] Interruption and resumption of DOACs for surgery and invasive procedures is generally based on inferences from the agents’ pharmacokinetics, although clinical trials reporting the effect of interruption strategies for dabigatran have been published.[154][155][156] An electronic application has been designed for use on smartphones, which can assist with timing of interruption of various anticoagulants for procedures.[157] For electrophysiology device procedures, a strategy of interrupting versus continuing DOACs resulted with similar outcomes between groups.[158]
A strategy for periprocedural anticoagulation assessment is presented here, based on published guidelines with suggested modifications by the authors based on additional data.[17][18][69][87][107][147][151][159][160][Figure caption and citation for the preceding image starts]: Perioperative management of vitamin K antagonists (warfarin)Adapted by BMJ Knowledge Centre from Douketis JD et al. Chest. 2022 Nov;162(5):e207-43; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Perioperative management of direct oral anticoagulants (DOACs)Adapted by BMJ Knowledge Centre from Douketis JD et al. Chest. 2022 Nov;162(5):e207-43; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Perioperative management of direct oral anticoagulants (DOACs)Adapted by BMJ Knowledge Centre from Douketis JD et al. Chest. 2022 Nov;162(5):e207-43; used with permission [Citation ends].
High thrombosis risk is defined as follows:[18]
Mechanical heart valve patients:
Any mechanical mitral valve
Caged ball or tilting disc valve in mitral/aortic position
Recent (<3 months) stroke or TIA.
Atrial fibrillation patients:
CHADS2 score of 5 or 6
CHA₂DS₂-VASc score of 7 or more
Recent (<3 months) stroke or TIA
Rheumatic valve disease.
Venous thromboembolism (VTE) patients:
Recent (<3 months and especially 1 month) VTE
Severe thrombophilia (deficiency of protein C, protein S or antithrombin, homozygous factor V Leiden or prothrombin gene mutation or double heterozygous for each mutation, multiple thrombophilias)
Antiphospholipid antibodies
Associated with vena cava filter
Active cancer associated with high VTE risk (including pancreatic cancer, myeloproliferative disorders, primary brain cancer, gastric cancer, esophageal cancer).
Bleeding risk of the surgical procedure should be assessed. If the bleeding risk is minimal (e.g., selected ocular, dental, and dermatologic procedures) or data support continued anticoagulation (e.g., pacemaker placement, catheter ablation for atrial fibrillation), continue anticoagulant at the usual dose. For warfarin, make sure that the INR is not above the target range, and if it is, the procedure should be delayed until the INR is in the target range. If the bleeding risk is other than minimal, the decision depends on the specific anticoagulant being used.
Unfractionated heparin (UFH)
If a patient is receiving UFH, this should be held 4-6 hours prior to procedure start time, and a normal activated partial thromboplastin time (aPTT) verified before surgery is started.[151] It can be resumed once hemostasis is secure and risk of post-procedure bleeding has abated (i.e., commonly 24-72 hours post-procedure). Heparin is generally resumed at the last rate of infusion the patient received pre-operatively and without an initial bolus.
Warfarin
Low risk of thrombosis: do not use bridging low molecular weight heparin (LMWH). Hold warfarin for 5-6 days prior to procedure. Some practitioners choose to verify that the INR is acceptable (generally ≤1.4) prior to surgery; but an observational study of 224 patients where warfarin was interrupted 5 days before surgery found that only 7% had an INR of 1.5 or greater before the procedure.[161]
Intermediate risk of thrombosis: guidelines advise against the use of low molecular weight heparin during warfarin interruption. Management is the same as for low risk of thrombosis.
High risk of thrombosis: bridging with parenteral anticoagulant is suggested. Hold warfarin for 5-6 days prior to procedure. Begin full-dose LMWH 3 days prior to procedure. Stop LMWH 24 hours before planned procedure start time (the last preprocedure dose of LMWH should be one-half of the full daily dose if once-daily enoxaparin or dalteparin is being used). Some practitioners choose to verify that the INR is acceptable (generally ≤1.4) prior to surgery.
Post-procedure resumption: because there is a delay of several days before warfarin exerts a substantial anticoagulant effect, it is generally resumed the evening following the procedure (or as soon as the patient is able to take oral medications) at the patient’s usual dose. LMWH exerts an immediate effect and timing of post-procedure resumption depends on the bleeding risk of the procedure. Resume LMWH postoperatively once hemostasis is secure and risk of post-procedure bleeding has abated (commonly 24 hours after procedures with low bleeding risk, and 48-72 hours for higher bleeding risk procedures). Prophylactic dose LMWH can be considered earlier if full-dose LMWH is delayed. The decision to resume LMWH should always be made in conjunction with the proceduralist to assure that an adequate assessment of bleeding risk has been undertaken. Discontinue LMWH when INR has returned to the target range.
Peri-procedural warfarin dosing and timing: an American College of Chest Physicians guideline has made specific recommendations regarding warfarin interruption. These include: holding warfarin for ≥5 days before procedures requiring interruption; refraining from vitamin K administration when INR is elevated 1-2 days prior to the procedure; resuming warfarin within 24 hours following the procedure; and resuming at the patient’s usual pre-procedure dose (rather than starting with an increased dose).[18]
Direct oral anticoagulants (DOACs)
Low/moderate bleeding risk procedures: hold drug for approximately three DOAC half-lives prior to procedure (adjust half-life estimate for renal function) and resume drug approximately 24 hours after procedure and adequate achievement of post-procedural hemostasis.
High bleeding risk procedures: hold drug for four to five DOAC half-lives prior to procedure (adjust half-life estimate for renal function) and resume drug approximately 48 to 72 hours after procedure and adequate achievement of post-procedural hemostasis.[18][147]
Reversal agents
Guidelines recommend against the use of pharmacologic anticoagulation reversal for elective surgery or procedures. Consideration can be given to use of these agents (see Reversal agents below) for emergency surgeries which cannot be safely postponed to allow anticoagulant effect to abate.[17][18][162][163]
[Figure caption and citation for the preceding image starts]: Perioperative management of direct oral anticoagulants (DOACs)Adapted by BMJ Knowledge Centre from Douketis JD et al. Chest. 2022 Nov;162(5):e207-43; used with permission [Citation ends].
Online tools which assist with all aspects of peri-procedural anticoagulation are available.
Management of anticoagulation in the peri-procedural period Opens in new window
University of Michigan: MAQI2 anticoagulation toolkit Opens in new window
An electronic application has been designed for use on smartphones which can assist with timing of interruption of various anticoagulants for procedures.[157]
Use of anticoagulants during pregnancy is associated with potential fetal and maternal harm. However, objective evidence regarding the potential harms and the burden of using these agents in pregnancy is limited.[164][165]
Vitamin K antagonists, such as warfarin, are considered teratogenic. Use during pregnancy can result in fetal bleeding, pregnancy loss, and impaired neurological development. However, if discontinued prior to the sixth week of gestation, the risk of fetal harm is thought to be minimal.[166][167][168][169][170]
Pregnant women with mechanical heart valves represent a special challenge, as data on the efficacy of anticoagulants other than warfarin to prevent valve thrombosis is limited. In one meta-analysis, continuing warfarin during pregnancy resulted in fewer maternal complications, but also fewer live births, compared to low molecular weight heparin (LMWH). The safety of unfractionated heparin (UFH) and lower doses of warfarin could not be confirmed.[171] UFH and LMWH cross the placenta, but are considered safe to use in pregnancy.[168][172][173][174][175] LMWH appears to be safer than UFH, with lower rates of hemorrhage, heparin-induced thrombocytopenia, and osteopenia/osteoporosis reported.[176][177][178][179][180][181][182][183][184] Guidelines and evidence-based reviews recommend LMWH as the preferred anticoagulant in pregnancy.[31][164][185][186][187][188]
There are reports of fondaparinux being used safely in pregnancy, despite the fact that it may cross the placenta.[189][190][191][192][193]
Pregnant women were excluded from trials assessing the safety and efficacy of direct oral anticoagulants and available evidence suggests risks for fetal abnormalities and miscarriage.[194] Therefore, these agents are not recommended.
The vitamin K antagonists warfarin and acenocoumarol are not detectable in breast milk.[195][196][197][198] Warfarin is, therefore, considered safe in breast-feeding. The product package insert for warfarin still cautions use, given this data was not from a high-quality study, and recommends assessing risk/benefit and monitoring the infant for bleeding/bruising.
Low molecular weight heparin and unfractionated heparin are also considered safe.[164][185][186][199]
Fondaparinux should be used with caution in breast-feeding as the manufacturer has reported excretion of the drug in lactating rats, although significant absorption by the infant is thought to be unlikely.[200][201]
Evidence supporting the safety of direct oral anticoagulants (DOACs) in breast-feeding does not exist, and the manufacturers recommend against using these medications. All DOACs are excreted in breast milk to varying degrees.[202]
Thromboembolism in pediatric patients is relatively rare compared to adults; therefore, there is limited evidence regarding the management of anticoagulation in this patient group. As the physiology and pharmacokinetic response to anticoagulants differs in children, it is recommended that these patients are managed by a pediatric hematologist with expertise in anticoagulation.[203] It is likely that children metabolize and respond to anticoagulants differently.[204][205] There are also challenges in regards to vascular access, suitable formulations of drugs for children, dietary differences, and adherence.
Warfarin availability in tablet form limits dependable administration to young children.[206][207] The target INR in children is 2.5 (range 2.0 to 3.0), except in the setting of mechanical heart valves, where adherence to adult targets (INR target 3.0, range 2.5 to 3.5) is recommended.[33][132][203]
Low molecular weight heparin (LMWH) has advantages over warfarin and unfractionated heparin (UFH), including reduced monitoring, lack of interference with diet, reduced rate of heparin-induced thrombocytopenia, and, importantly in this patient group, a reduced risk of bone demineralization. However, the predictability of the anticoagulant effect with weight-based dosing is less certain in children, presumably due to unreliable plasma drug binding.[208] For this reason, it is recommended that the drug is monitored for therapeutic effect and titrated to a target anti-Xa activity range of 0.5 to 1.0 units/mL.[203][209]
UFH should be titrated to an active partial thromboplastin time (aPTT) representative of an anti-Xa level of 0.35 to 0.7 units/mL, or titrated according to anti-Xa levels, rather than to aPTT; many pediatric institutions favor management by anti-Xa activity, due to less certain correlation of aPTT with heparin levels in children.[210]
There are few studies on the use of direct oral anticoagulants (DOACs) in children. One RCT compared age- and weight-adjusted dabigatran with standard of care (LMWH, UFH, vitamin K antagonist, or fondaparinux) for relatively short-term anticoagulation (average duration <90 days). Dabigatran was associated with similar efficacy, rates of bleeding and adverse events.[211] Another study of rivaroxaban in children with venous thromboembolism showed similar outcomes to standard of care, with equally low bleeding and thrombotic outcomes, though with a reduced thrombotic burden in patients receiving rivaroxaban.[212] A trial of apixaban in children with congenital or acquired heart disease reported reassuring rates of thrombosis and bleeding compared with LMWH.[213] Several other trials of DOACs in children for a variety of indications are underway, and although initial results have been encouraging, results of larger phase 3 trials will better inform the standard of care in children.[214][215][216][217]
Increasing age is associated with an increased risk of disease for which anticoagulation therapy is indicated, but also with an increasing risk of bleeding from anticoagulant therapy. Anticoagulants appear to be under-utilized in patients who would benefit from antithrombotic therapy and advanced age is often cited as a relative contraindication.
The combined benefit attributable to anticoagulation in conjunction with the rate of adverse outcomes attributable to anticoagulation in older people has been assessed, and while the benefit of anticoagulation is dependent on individual patient risk factors for thrombosis and risk of bleeding, benefit generally outweighs risk.[61][218][219][220][221][222]
Risk factors for bleeding in older people include: ground level falls; advancing age; poorly-controlled hypertension; history of major bleeding; history of poor anticoagulation management; concomitant antiplatelet therapy; and excessive alcohol ingestion.[58][61][62][65][66][67][138][223][224] These risk factors have been assessed for predictive utility in assessing patients who would benefit from anticoagulation therapy. Formalized risk assessment tools that take patient age into consideration, in conjunction with other comorbidities that affect the risk of anticoagulation, include:[62][67][68]
As increasing age is a significant risk factor for adverse events among patients with an indication for anticoagulation, tools for estimating the risk of outcome events of arterial and venous thrombosis among patients with atrial fibrillation, mechanical heart valves, and venous thromboembolism (VTE) exist:[32][33][34]
The risk of major bleeding in older patients taking direct oral anticoagulants (DOACs) for VTE or atrial fibrillation is lower compared to vitamin K antagonists.[119][225][226][227] This means that older patients who would not have previously been considered candidates for therapy may be considered suitable for DOAC therapy.[49][228] It also means that the bleeding risk associated with extended treatment in these patients may now be considered acceptable.[45][48][50]
A dose adjustment may be required in patients with renal impairment, depending on the specific anticoagulant used. Some agents are not recommended in patients with certain degrees of renal impairment. For some agents, dose adjustments differ according to the indication.
Warfarin: renal dysfunction may influence response; however, no dose adjustment is required and dose is based on INR as usual.[110] Warfarin is not directly excreted renally; however, patients with severe renal dysfunction have elevated risk for bleeding while on warfarin therapy.[229]
Unfractionated heparin (UFH): may be used in renal impairment and end-stage kidney disease with no dose adjustment required as long as the activated partial thromboplastin time (aPTT) is monitored appropriately and the dose adjusted according to the aPTT.[206][230][231]
Low molecular weight heparin (LMWH): usual therapeutic doses are not recommended in patients with significant renal impairment unless the trough anti-Xa effect is monitored before subsequent doses.[232][233][234][235] Specific dose adjustments based on creatinine clearance are recommended and depend on which LMWH is used.[236][237][238]
Fondaparinux: caution is recommended in patients with a creatinine clearance of 30-50 mL/min, and use is contraindicated if creatinine clearance is <30 mL/minute.[110][201]
Apixaban: for the indication of stroke prevention in atrial fibrillation, a dose reduction is recommended in patients with two or more of the following factors: serum creatinine ≥1.5 mg/dL; age ≥80 years; or body weight ≤60 kg. No dose reduction is recommended in patients with end-stage kidney disease who are on hemodialysis, although very few dialysis patients were included in the kinetic study on which this statement is based. For treatment of venous thromboembolism (VTE), apixaban is approved for use without dose adjustment, although serum concentrations are higher in patients with severe renal dysfunction. Studies evaluating safety of apixaban in patients with severe renal dysfunction suggest that it is a reasonable alternative to warfarin and may be associated with a lower risk of bleeding.[229][239][240][241]
Edoxaban: use is not recommended in severe renal impairment (creatinine clearance <15 mL/min). A dose reduction is recommended for patients with nonvalvular atrial fibrillation who have a creatinine clearance of 15-50 mL/minute and for patients with VTE who have a creatinine clearance of 15-50 mL/minute, a body weight ≤60 kg, or are also receiving P-glycoprotein inhibitors. As edoxaban is so heavily dependent on renal excretion, serum levels are markedly reduced in patients with creatinine clearance >95 mL/minute and is therefore not recommended in these patients.[14][242][243][244]
Rivaroxaban: a dose reduction is recommended in patients with nonvalvular atrial fibrillation and a creatinine clearance of <50 mL/min. While patients with an estimated creatinine clearance of <15 mL/min were not studied in the atrial fibrillation trials, the approved prescribing information suggests that drug exposure should be similar. Efficacy in patients on dialysis is uncertain. Rivaroxaban is not recommended in patients with VTE and a creatinine clearance of <30 mL/min.[13][245]
Dabigatran: the direct oral anticoagulant that is most dependent on renal function.[246] For stroke prevention in patients with nonvalvular atrial fibrillation, a dose reduction is recommended in patients with severe renal impairment (creatinine clearance 15-30 mL/min); there are no dose recommendations in patients with a creatinine clearance <15 mL/min or those on dialysis. For the treatment or prevention of deep vein thrombosis and pulmonary embolism, there are no dose recommendations for patients with a creatinine clearance <30 mL/min or those on dialysis. In patients with renal impairment who are also on a P-glycoprotein inhibitor, use should either be avoided or the dose reduced depending on the indication for treatment.[15]
A dose adjustment may be required in patients with hepatic impairment, depending on the specific anticoagulant used.
Warfarin: metabolism may be affected by hepatic impairment, which may increase response to warfarin by inhibiting clotting factor production and decreasing warfarin metabolism.[102][110] Patients may require a lower starting dose and slower titration of the dose.
Unfractionated heparin: activity requires presence of antithrombin, which is produced in the liver; however, a dose adjustment is not defined.[231]
Low molecular weight heparin (LMWH): use with caution in patients with hepatic impairment, although no specific dose adjustments are defined.[237][238] LMWH is suggested as the preferred agent in patients with severe liver disease who require anticoagulant therapy.[247]
Fondaparinux: no dose adjustment recommended for Child-Pugh class A (mild impairment) or Child-Pugh class B (moderate impairment). There are no data for patients with severe impairment.[201]
Apixaban: no dose adjustment recommended in Child-Pugh class A and no recommendations for Child-Pugh class B, but there may be an increased risk of bleeding; not recommended in Child-Pugh class C (severe impairment).[16]
Edoxaban: no dose adjustment required in Child-Pugh class A, but not recommended in Child-Pugh class B or C.[14]
Dabigatran: use with caution in Child-Pugh class B and not recommended in Child-Pugh class C.[15]
The greatest risk for bleeding is in patients who require dual antiplatelet therapy in conjunction with anticoagulation (also known as triple antithrombotic therapy), though this strategy is increasingly rare; when used, triple antithrombotic therapy should be continued for the shortest possible period and only in high thrombotic risk patients.[19] The concomitant use of antiplatelet agents and oral anticoagulation occurs primarily in patients with atherosclerotic heart disease and atrial fibrillation, with 30% of atrial fibrillation patients requiring percutaneous coronary intervention (PCI).[224][249][250] After PCI, the duration of dual antiplatelet therapy varies from 4 weeks to a minimum of 6-12 weeks.[19] Dual antiplatelet therapy has been found to be superior to aspirin plus oral anticoagulation in patients undergoing PCI.[251][252][253] Studies continue to focus on duration of the antiplatelet component of therapy following elective PCI, and evidence is mounting regarding use of shorter durations of dual or triple antithrombotic therapy before reverting to treatment with a single antiplatelet agent and an anticoagulant.[19][254][255][256] Present guidelines recommend that all patients with unstable angina or ST-elevation myocardial infarction receive aspirin indefinitely and dual antiplatelet therapy (e.g., aspirin plus clopidogrel) for up to 12 months.[257] Newer data is informing the use of the safest individual agents in combination regimens.[256]
Oral anticoagulation in combination with antiplatelet therapy is associated with an annual risk of fatal and nonfatal bleeding of 4% to 14%.[224] A small study reported a bleeding rate of 9.2% among patients receiving triple antithrombotic therapy.[258] The risk of major bleeding with warfarin in conjunction with antiplatelet therapy has been reported to be between 4.7% and 6.2%.[259][260] It appears that the first month of triple antithrombotic therapy is associated with the highest risk of bleeding occurring.[259]
There are no clear recommendations for a particular anticoagulant or antiplatelet agent in patients who require concomitant therapy.[223][224][261] However, various interventions may lower the risk of bleeding in patients who require triple antithrombotic therapy. Maintaining an INR of 2.0 to 2.5 in patients may be associated with a lower risk of bleeding compared to a target INR of >2.6.[252] More frequent monitoring of the INR may also be helpful. One trial suggested that apixaban combined with clopidogrel resulted in similar efficacy but less bleeding than a regimen of either aspirin, warfarin or both.[256]
Low-dose aspirin (i.e., <100 mg/day) and clopidogrel are the preferred antiplatelet agents for patients who require triple antithrombotic therapy. If, or when, anticoagulation plus a single antiplatelet is indicated, clopidogrel should be considered instead of aspirin.[262] Use of clopidogrel without aspirin was associated with a significant reduction in bleeding complications and no increase in the rate of thrombotic events.[224]
Recommendations from both the 2024 European Society of Cardiology (ESC) and the 2020 American College of Cardiology (ACC) atrial fibrillation guidelines for patients undergoing PCI, with either acute coronary syndrome or stable coronary syndromes suggest a short course of aspirin and continuation of clopidogrel (the preferable P2Y12 inhibitor) with an oral anticoagulant for 6 to 12 months depending on risk of stent thrombosis and bleeding. Combining anticoagulant and antiplatelet therapies is not recommended without a compelling indication for both classes of drugs.[19][263] When combined, the second agent should be continued for the shortest period needed based on the indication.
Gastroprotective therapy may be considered in patients with a history of gastrointestinal hemorrhage. The need for triple antithrombotic therapy should be frequently reassessed.
All anticoagulants share the principal adverse effect of bleeding, as well as other adverse effects unique to the medication class and individual agents. Important nonbleeding related adverse effects are highlighted below; however, the full prescribing information of an individual drug should be consulted for a complete list.
Warfarin
Severe skin necrosis syndrome: can occur in susceptible individuals, especially those with hereditary deficiency of protein C. Risk is increased when higher doses of warfarin are used at the initiation of therapy.[130] The presence of heparin-induced thrombocytopenia (HIT) increases the risk of skin necrosis if warfarin is continued during the active phase of the disease (i.e., while thrombocytopenia is present).[264] Also associated with a rare vascular complication called purple toe syndrome.[265]
Osteoporosis: may be associated with osteoporosis, although the evidence for this is conflicting.[266]
Unfractionated heparin
HIT: an immunologic complication that results in aggressive thrombosis in arteries and/or veins, which can result in limb amputation, severe organ dysfunction, and death. Risk is dependent on the patient’s circumstances and the dose of heparin. Risk is highest in orthopedic surgery patients and lowest in obstetric patients.[95] It is often over-diagnosed; diagnosis should be achieved with careful adherence to evidence-based diagnostic criteria.[177] Treatment consists of absolute avoidance of heparinoids at any dose, and use of an alternate anticoagulant such as argatroban until the platelet count recovers. Heparin use is contraindicated in patients with a previous history of HIT.
Skin necrosis syndrome: very rare and may actually overlap with HIT.[265]
Osteoporosis: prolonged use is associated with osteoporosis.[266]
Allergic reactions: severe allergic reactions were reported following administration of heparin contaminated with over-sulfated chondroitin sulfate.[267]
Low molecular weight heparin (LMWH)
HIT: can also occur with LMWH, but is less frequent compared to unfractionated heparin.[95]
Dabigatran
Acute coronary events: has been associated with an increased risk of acute coronary events in some studies, although others have failed to show this association.[268][269]
Can cause dyspepsia.
Bleeding is usually easily detected by patients, though it can be subtle or asymptomatic. The definition of bleeding and quantification of its severity in clinical trials varies; however, the International Society on Thrombosis and Haemostasis has suggested the following categories:[270]
Fatal bleeding
Major bleeding: bleeding into a critical organ (including the: central nervous system; retina; retroperitoneal, intra‐articular, or pericardial spaces; or intramuscular compartment with compartment syndrome), bleeding that leads to surgery or requires other invasive therapies to control it, or overt bleeding that results in the transfusion of at least 2 units of packed red blood cells.
Clinically relevant nonmajor bleeding: bleeding that results in evaluation or treatment by a healthcare provider that does not meet the criteria for major bleeding.
A similar set of bleeding definitions was created for surgical patients in the postoperative period.[271]
The risk of bleeding is dependent on several factors, including characteristics of the patient, extrinsic circumstances and the specific anticoagulant. Generally, higher doses are associated with a greater risk of bleeding compared to lower doses, and prophylactic doses are associated with a lower risk of bleeding compared to treatment doses.
For warfarin, higher INR values predict a higher risk of bleeding. While there is some variation between studies, based on the indication being studied, the bleeding risk of direct oral anticoagulants (DOACs) is similar to, or lower than, that of warfarin. The site of bleeding also depends on the specific anticoagulant used. DOACs are more likely to cause gastrointestinal bleeding compared to warfarin, although the specific risk of individual drugs within this class may differ.[272] One study analyzed the gastrointestinal bleeding risk of DOACs and compared differences among these drugs in age-related risk of gastrointestinal bleeding. The overall incidence of gastrointestinal bleed events was 2.74/100 patient-years with rivaroxaban versus 2.02/100 patient-years with dabigatran; 1.38/100 patient-years with apixaban versus 2.73/100 patient-years with dabigatran; 1.34/100 patient-years with apixaban versus 3.54/100 patient-years with rivaroxaban.[273] The study found that apixaban had the most favorable gastrointestinal safety profile and rivaroxaban the least favorable.
DOACs are less likely to cause intracranial hemorrhage or fatal bleeding compared to warfarin.[274][275]
Parenteral anticoagulants have the highest risk of intramuscular or rectus sheath hematoma.[276]
Oral factor Xa inhibitors have a higher risk of gastrointestinal bleeding when compared to low molecular weight heparin in patients with cancer. This risk is largely confined to patients with luminal gastrointestinal malignancies and likely varies by agent.[277]
Management of bleeding during anticoagulation requires a comprehensive evaluation and various interventions to mitigate the risk of morbidity and mortality. Elements of a comprehensive management strategy include a number of general interventions that are applicable regardless of the agent the patient is taking, along with agent-specific reversal strategies when indicated and available. Source control and supportive care are the mainstay of management and should not be delayed. Use of a reversal agent does not replace the need for these measures. Specific and nonspecific reversal agents have risks, which should be balanced against predicted benefit before deciding to employ these.[278]
The following algorithm details the approach to managing an anticoagulated patient with acute hemorrhage.[69][279][280][281][282]
[Figure caption and citation for the preceding image starts]: Approach to the management of acute hemorrhage in an anticoagulated patientProduced by SM Stevens, MD and SC Woller, MD; used with permission [Citation ends].
General measures and blood products
Patients who are bleeding should be assessed for the presence of hemodynamic consequences related to bleeding, and provided with supportive care to maintain organ perfusion and oxygenation. Supportive care includes intravenous fluids, transfusion of packed red blood cells, and in the case of massive bleeding, replacement of lost platelets and coagulation proteins with platelet and plasma infusions.
Source control
In many instances, interventions directed at the source of bleeding may be used to stop or reduce ongoing blood loss. These can include pressure on external wounds, endoscopic interventions for gastrointestinal sources (and occasionally respiratory and genitourinary tract sources) of bleeding, interventional radiology procedures (e.g., embolization of vessels which perfuse the bleeding location), and surgical control of bleeding lesions.
Nonspecific prohemostatic agents
Antifibrinolytic agents act by inhibiting the breakdown of existing fibrin thrombi, and may reduce bleeding without specifically inhibiting the effect of anticoagulants. Tranexamic acid is the best studied of these agents, and has been evaluated to control bleeding following trauma, as well as to treat heavy uterine bleeding.[283]
Specific anticoagulant reversal agents may also be required.
After bleeding has been controlled, the patient should be assessed and a decision made whether to resume anticoagulation, and if so, when. There is limited evidence available to guide these decisions. Data from registries suggest that outcomes, including stroke and mortality, are better if anticoagulation is resumed following a major gastrointestinal hemorrhage rather than if anticoagulation is permanently stopped. The best outcome may be obtained if anticoagulation is resumed 1-2 weeks following treatment of a bleeding episode.[284][285] Similar data support the re-introduction of anticoagulant therapy following an intracranial bleed; however, this depends on the type of hemorrhage, as traumatic bleeds and deep intracerebral bleeds due to hypertension tend to have a lower risk of re-bleeding.[286][287][288] Choice of anticoagulant agent, timing of resumption, and which patients should and should not resume anticoagulation is less clear. While precise methods of predicting the risk-benefit balance of reinstituting anticoagulants is unavailable, the American Heart Association and American Stroke Association suggest considering the following factors to help inform decision-making:
lobar location of the initial intracranial hemorrhage
older age
presence, number, and lobar location of microbleeds on MRI
presence of disseminated cortical superficial siderosis on MRI
poorly controlled hypertension
Asian or Black race; and
presence of apolipoprotein ApoE-2 or ApoE-4 alleles.[282]
The decision to resume anticoagulation should be made in conjunction with specialists involved in managing the bleeding complication, and the patient’s values and preferences should be taken into account.
Reversing the effect of an anticoagulant is often part of the management strategy for bleeding; however, the use of reversal agents should always be complementary to other measures (e.g., stopping anticoagulant, source control, blood products). Restoring the coagulation capacity of the blood does not repair injuries or lesions that are actively bleeding, improve hemodynamic compromise, or restore lost blood. Therefore, appropriate supportive care and source control of the bleeding are the top priorities. Reversing the effect of an anticoagulant may also be required for other reasons, including the need for urgent or emergency invasive procedures, the use of thrombolytic agents for acute stroke or acute coronary syndrome, or to counteract the effects of a supratherapeutic level of anticoagulant. All reversal agents have risks, which should be balanced against predicted benefit before deciding to employ these.
The choice of reversal agent depends on the specific anticoagulant used. Dosing also depends on the specific anticoagulant used, as well as the dose and time since the agent was last administered. Each reversal agent has unique risks and adverse effects; however, all agents share a common risk of possible thrombosis. Reversal agents should, therefore, only be used when the benefits outweigh the risks.
No reversal agents exist for argatroban, bivalirudin, or desirudin. However, due to their short half-lives, discontinuing these agents results in rapid elimination of their anticoagulant effect.
Protamine sulfate is the agent of choice to reverse the effect of unfractionated heparin and low molecular weight heparin (LMWH). This drug binds to heparin to form a stable salt, which results in rapid reversal of the anticoagulant effect of heparin.[80] Protamine sulfate is given intravenously, and the dose is based on the number of units of heparin that the patient has received and the timing of dosing. The dose is less certain if unfractionated heparin was administered subcutaneously, and an infusion may be required. For intravenous unfractionated heparin, the effect of reversal can be assessed by measuring the activated partial thromboplastin time (aPTT). Protamine sulfate is thought to partially neutralize the effect of LMWH, but there is little clinical evidence to demonstrate that reversal has a beneficial impact on bleeding. Dosing is based on the dose of LMWH and when the last dose was received, taking into consideration renal dysfunction, which may prolong activity of LMWH. The most serious adverse effect is anaphylaxis, and a known allergy to fish (from which protamine is derived) may predict this risk.[289]
Recombinant coagulation factor Xa (andexanet alfa) may also reverse the effect of heparins, though it is not approved for this indication and dosing strategy is unknown.[290]
Vitamin K (phytonadione) reverses the effect of warfarin by interfering with its mechanism of action, restoring the effective synthesis of vitamin K-dependent coagulation factors II, VII, IX, and X. Its effect is not immediate as it requires synthesis of new coagulant proteins. Vitamin K has a faster onset of action when given by intravenous infusion, with its effect starting approximately 2 hours after administration and the full effect of its dose achieved within 24 hours.[130] Oral administration has similar efficacy to intravenous administration, but the full effect of the dose is not achieved until approximately 36 hours after administration, and a higher dose is needed to achieve the same effect as an intravenous dose.[130] Dose depends on the pre-dose INR, whether complete or partial reversal is required, and the indication for reversal. In life-threatening bleeding, full reversal is usually required and a high dose should be given.[291] If intravenous dosing is chosen for cases of less serious bleeding episodes, and subsequent resumption of anticoagulation with warfarin is anticipated, smaller doses should be used.[292][293] Low doses of oral vitamin K are used in patients with supratherapeutic INR values when the goal is to restore the INR to the target range.[107] For patients with INRs of >4.5 but <10 and without clinically relevant bleeding, a systematic review found no evidence for benefits with use of vitamin K and the American Society of Hematology guidelines suggest temporary cessation of warfarin alone without the addition of vitamin K.[139][294]
Anaphylaxis with intravenous infusions is uncommon but can be mitigated by giving the infusion slowly in a dilute solution.[130] Vitamin K does not appear to increase the risk of thrombosis; therefore, the risk is due to removing the protective effect of the anticoagulant.
In patients who are bleeding or those who require very rapid reversal, either fresh frozen plasma (FFP) or prothrombin complex concentrates (PCCs) are preferred to vitamin K; PCCs are preferred over FFP in most situations in life-threatening or major bleeding.[130][292] However, as these agents tend to have shorter serum half-lives compared to warfarin, they are usually combined with vitamin K to prevent the recurrence of the anticoagulant effect of warfarin that has not yet been eliminated.[291][293]
FFP restores coagulation by replacing coagulation factors. While it has been the agent used principally for emergency reversal in the US, it has several shortcomings.[295][296] As it must be thawed and cross-matched to the recipient, it takes significant time to administer. Additionally, a very large volume of plasma is required to substantially reduce the INR, and the volume needed may preclude practical use.[292] Even at very high volumes, it is unlikely to have further effect on the INR once the INR reaches approximately 1.5.[297] The dose depends on the pre-dose INR. Dose calculators are available but are relatively complex.[292][298] FFP can cause transfusion reactions, including life-threatening reactions, in the presence of an incorrect cross-match or unusual antibodies, and infections transmitted from plasma donors or contamination. Higher volumes of infused plasma may result in transfusion-related circulatory overload and transfusion-associated acute lung injury. Thrombosis may also occur.
Guidelines have suggested use of PCCs in preference to FFP, and the use of FFP appears to be decreasing.[130][282][295] PCCs are coagulation factor products which are prepared from pooled plasma and replace the dysfunctional coagulation proteins that result from warfarin therapy. PCCs come in several forms, including 3-factor preparations (which contain factors II, IX, and X) and 4-factor preparations (which also contain factor VII).[299] Standard PCCs contain the proteins in their inactivated state. Activated PCCs contain activated, rather than inactivated, factor VII. Due to their powdered formulation and lack of need for cross-matching, PCCs can be given much more rapidly than FFP, carry little, if any, risk of transfusion reactions, and are infused in small volumes. These agents were developed to address bleeding complications in patients with hemophilia, and use for reversal of warfarin was originally not approved. An inactivated 4-factor PCC (4F-PCC) was approved by the Food and Drug Administration (FDA) specifically to reverse the effect of warfarin. PCCs are favored over FFP for warfarin reversal in patients with serious or life-threatening bleeding in evidence-based guidelines.[130] One systematic review found that the use of 4F-PCC for warfarin reversal is superior to FFP in normalizing the INR, with no difference in the rate of thrombosis.[300] The main risk associated with PCCs is arterial or venous thrombosis. In one study, the rate of thrombosis was reported to be 3.9%.[301] 4F-PCC should be avoided in patients with a history of heparin-induced thrombocytopenia, as it contains heparin.
Recombinant activated factor VII (rVIIa) is a concentrate of factor VII in its activated form that has been approved by the FDA to address serious and life-threatening bleeding in patients with hemophilia. Warfarin reversal is among a number of off-label uses for which rVIIa has been administered. One systematic review and meta-analysis did not show a survival benefit for use of rVIIa for off-label indications, but did detect a risk of thromboembolism following administration.[302] Use of rVIIa has been suggested for life-threatening bleeding in one major guideline, but analysis of use according to available treatment guidelines did not lead to improved mortality in a registry.[130][303]
When introduced, DOACs lacked any proven reversal agent. Activated, inactivated, 3-factor, and 4-factor prothrombin complex concentrates (4F-PCCs), as well as recombinant activated factor VII (rVIIa) have all been studied as possible reversal agents for dabigatran, but most studies have been performed using in vitro specimens, and the few in vivo studies available have been confined to nonbleeding, healthy volunteers. Study endpoints have been limited to assessment of various coagulation parameters. A meta-analysis of nonspecific reversal agents for DOACs indicated that trial results have been limited to laboratory parameters, and have not been consistent.[300]
The role of PCCs in patients receiving dabigatran has been displaced by the approval of idarucizumab, a specific reversal agent for dabigatran. Idarucizumab is a full humanized antibody fragment (Fab) directed against dabigatran which nullifies its anticoagulant effect. It is administered by intravenous infusion. In an open-label trial, 503 patients taking dabigatran who experienced either acute bleeding or required urgent surgery received idarucizumab. The median time to cessation of bleeding was 2.5 hours, and operative hemostasis was assessed as normal in 93.4% of surgeries. Approximately 7% of patients sustained a new thrombosis within 90 days.[304] Immunogenicity to the Fab fragment may also develop; however, the implications of this are not yet clear. Investigational agents are also in development. In addition to reversal agents, recent ingestion of dabigatran may be mitigated by administration of charcoal. Hemodialysis is also an option for removal of dabigatran.[305]
Recombinant coagulation factor Xa (andexanet alfa) is a bioengineered analog of factor Xa, which acts as a decoy target for factor Xa inhibitors and has no direct effect on the coagulation cascade. It has been approved by the FDA for patients treated with rivaroxaban and apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. According to its mechanism of action, it would be predicted to reverse the effects of other medications that affect factor Xa (edoxaban, fondaparinux, LMWH) but it is not approved for use to reverse the effect of these drugs, and dosing is unclear. Recombinant coagulation factor Xa (andexanet alfa) received approval through an accelerated approval process, based on limited data.[306] Additional studies have since been published.[307][308] The ANNEXA-I study, evaluating andexanet alfa compared to usual care in patients with intracerebral hemorrhage, compared patients with intracranial hemorrhage who had received apixaban within 15 hours of diagnosis of the bleeding episode. A total of 263 patients received recombinant coagulation factor Xa (andexanet alfa) vs. 267 randomized to usual care (which could include nonspecific prohemostatic agents, usually PCC). Use of recombinant coagulation factor Xa (andexanet alfa) was associated with less hematoma expansion but at the cost of nearly double the rate of thrombotic events.[309]
A small number of in vitro and in vivo studies have been performed with PCCs and rVIIa; however, results have been conflicting. No prospective comparative trials exist for recombinant coagulation factor Xa (andexanet alfa) and 4F-PCCs, the two reversal agents most likely to produce effective hemostasis. Therefore, the decision to use one over the other must carefully weigh the benefits of reversal against the risk of thrombosis. Previous guidelines and guidance statements have supported this choice.[87][310] Though newer guidelines give preference to recombinant coagulation factor Xa (andexanet alfa), if it is available.[282]
For the currently available DOACs, society guidance statements have recommended situations in which a specific antidote should be used, considered, or avoided.[18][311][312]
Utilize an antidote for:
Life-threatening bleeding
Bleeding in a closed space or critical organ
Persistent bleeding despite local measures
Need for an urgent intervention that cannot be delayed or done while on the DOAC
Emergency surgery with high bleeding risk.
Consider use of an antidote for:
Need for or an urgent surgery or intervention in a patient with acute renal failure.
Do not utilize an antidote for:
Elective surgery
Gastrointestinal bleeding responsive to other measures
High drug levels without bleeding
Surgery that can be safely delayed until the DOAC clears.
[Figure caption and citation for the preceding image starts]: Management of anticoagulant-related hemorrhage. aPCC indicates activated prothrombin complex concentrate; DOAC, direct oral anticoagulant; ICH, intracerebral hemorrhage; INR, international normalized ratio; and PCC, prothrombin complex concentrate.Greenberg SM et al. Stroke. 2022;53:e282-361; used with permission [Citation ends].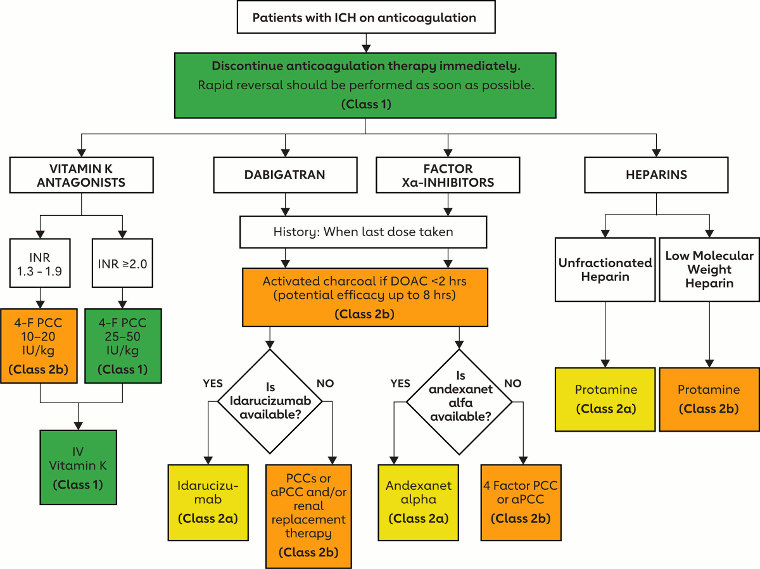
Pharmacology: inhibit vitamin K epoxide reductase complex resulting in synthesis of defective vitamin K-dependent clotting factors (II, VII, IX, and X). Warfarin is the only drug available in this class in the US.[102]
Indications: treatment and prophylaxis of acute venous thromboembolism (VTE); prevention of thromboembolic events in atrial fibrillation and/or prosthetic heart valve replacement; to reduce risk of death, recurrent myocardial infarction (MI), and thromboembolic events post MI.
Contraindications: malignant hypertension; major regional or spinal anesthesia; spinal puncture or any other invasive procedure where there is risk of uncontrolled bleeding; recent or planned invasive surgery that carries considerable bleeding risk; bleeding or hemorrhagic tendencies associated with chronic disease states; pregnancy (except in women with mechanical heart valves); unmonitored nonadherent patients; hypersensitivity to warfarin; or whenever the risk of bleeding outweighs the benefits of therapy.
Cautions: heparin-induced thrombocytopenia (do not use as initial therapy); hepatic impairment.
Drug interactions: more than 200 drugs interact with warfarin, as well as many foods and supplements (see below).
Adverse effects: minor and major bleeding is the most common adverse effect associated with chronic use; tissue necrosis and systemic emboli occur rarely.
Monitoring: INR.
Consult local drug formulary for full prescribing information and dose.
[Figure caption and citation for the preceding image starts]: Possible interactions with warfarin. This list is not exhaustive and a local drug formulary should be consulted before prescribing any drug in patients on warfarinCreated by the BMJ Evidence Centre (based on Coumadin® package insert) [Citation ends].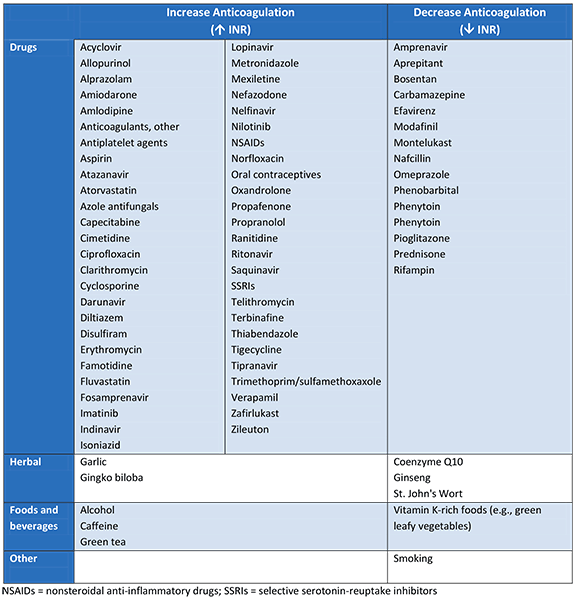
Pharmacology: directly inhibit all forms of thrombin. Dabigatran is currently the only drug available in this class.[15]
Indications: treatment and prophylaxis of acute venous thromboembolism (VTE); prevention of thromboembolic events in nonvalvular atrial fibrillation; prophylaxis of VTE after total hip replacement surgery. [
 ]
]
Contraindications: active bleeding; hypersensitivity to dabigatran; presence of mechanical heart valves (due to lack of efficacy and increased bleeding risk).[313]
Cautions: increased risk of thromboembolic events with premature cessation; risk of spinal or epidural hematoma with spinal or epidural anesthesia; hepatic and renal impairment.
Drug interactions: P-glycoprotein inhibitors and inducers; other drugs that can cause bleeding (e.g., antiplatelet agents, fibrinolytics, heparin, nonsteroidal anti-inflammatory drugs).
Adverse effects: bleeding is the most common adverse effect (see below).
In the RELY trial, atrial fibrillation patients were treated with dabigatran (110 mg twice daily or 150 mg twice daily) or dose-adjusted warfarin. There were significantly fewer major bleeds with the lower dose of dabigatran compared to warfarin, but no significant difference in bleeding with the higher dose. The higher dose of dabigatran resulted in more gastrointestinal bleeds than warfarin. Both doses of dabigatran resulted in fewer intracranial bleeds.[46]
In the RE-COVER and RE-COVER II trials, patients were randomized to receive dabigatran after treatment with a parenteral anticoagulant for 5 to 10 days, or a parenteral anticoagulant transitioned to warfarin. In the pooled analysis, there were no statistically significant differences in major bleeding or recurrent VTE.[39]
The RE-SONATE and RE-MEDY trial program evaluated the use of dabigatran compared to warfarin for extended VTE prophylaxis in patients who had already completed 3 to 18 months of prior anticoagulation. There was no statistically significant difference in major bleeding events in the warfarin or dabigatran arms, but dabigatran resulted in fewer clinically relevant nonmajor bleeds.[48]
Dabigatran was compared to enoxaparin in three trials investigating elective joint replacement. A meta-analysis of these trials showed noninferiority of dabigatran compared to enoxaparin for the prevention of VTE after joint replacement surgery, with a similar risk of bleeding.[314][315][316][317]
Consult local drug formulary for full prescribing information and dose.
Pharmacology: selectively inhibit factor Xa, including free and clot-bound factor Xa, and thus indirectly decrease thrombin formation and subsequent fibrin clot formation. This drug class includes apixaban, edoxaban, and rivaroxaban.[13][14][16][318]
Indications: prevention of thromboembolic events in nonvalvular atrial fibrillation (apixaban, rivaroxaban, and edoxaban); treatment of acute venous thromboembolism (VTE) (apixaban, rivaroxaban, and edoxaban); prevention of VTE following hip or knee surgery (apixaban and rivaroxaban) [
 ]
; secondary prevention of recurrent VTE (apixaban and rivaroxaban); VTE prophylaxis in acutely ill hospitalized adults who are at risk due to immobility or other risk factors for VTE, including, possibly, the post-discharge period, which may be up to 39 days in patients at high risk of VTE and low risk of bleeding (rivaroxaban); stable coronary artery disease or peripheral artery disease (rivaroxaban).
]
; secondary prevention of recurrent VTE (apixaban and rivaroxaban); VTE prophylaxis in acutely ill hospitalized adults who are at risk due to immobility or other risk factors for VTE, including, possibly, the post-discharge period, which may be up to 39 days in patients at high risk of VTE and low risk of bleeding (rivaroxaban); stable coronary artery disease or peripheral artery disease (rivaroxaban).
Contraindications: active bleeding.
Cautions: increased risk of thromboembolic events with premature cessation without transitioning to an alternative anticoagulant; risk of spinal or epidural hematoma with spinal or epidural anesthesia; hepatic and renal impairment (dose may need to be reduced or drug avoided depending on drug); mechanical heart valves; moderate-to-severe mitral stenosis (edoxaban); nonvalvular atrial fibrillation with creatinine clearance >95 mL/min (these patients have an increased risk of stroke with edoxaban compared with similar patients treated with warfarin).[87]
Drug interactions: strong inhibitors or inducers of P-glycoprotein and CYP3A4 (apixaban and rivaroxaban); strong P-glycoprotein inducers and inhibitors (edoxaban); other drugs that can cause bleeding (e.g., antiplatelet agents, fibrinolytics, heparin, nonsteroidal anti-inflammatory drugs).
Adverse effects: bleeding is the most common adverse effect (see below).
In the ARISTOTLE trial, which compared warfarin to apixaban in patients with nonvalvular atrial fibrillation, apixaban was significantly less likely to cause major bleeding.[42] In the AVERROES trial, which compared aspirin to apixaban in patients with nonvalvular atrial fibrillation, there was no significant difference in the risk of bleeding.[49] The AMPLIFY trial compared apixaban to warfarin/enoxaparin for the acute treatment of VTE. The relative risk of major bleeding was 0.31, favoring apixaban.[47] The AMPLIFY-EXT trial evaluated apixaban compared to placebo for extended secondary prevention of VTE (patients had completed 6 to 12 months of therapy with either apixaban or enoxaparin/warfarin in the AMPLIFY trial). The composite of major and clinically relevant nonmajor bleeding occurred in 27 patients (3.2%) versus 35 patients (4.3%) versus 22 patients (2.7%) in the apixaban (5 mg/day), apixaban (10 mg/day), and placebo groups, respectively.[50] The ADVANCE trial series looked at varying doses of enoxaparin compared to apixaban for the prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE) following hip or knee surgery. Apixaban did not differ from enoxaparin (40 mg/day) for bleeding outcomes, but was less likely to result in the composite endpoint of major bleeding and clinically relevant nonmajor bleeding than enoxaparin (60 mg/day).[319][320][321]
The ROCKET AF study enrolled patients with nonvalvular atrial fibrillation and randomized them to receive either warfarin or rivaroxaban. There was no significant difference in major bleeding between groups. There were significantly fewer (i.e., 0.8% versus 1.2%) intracranial hemorrhages in the rivaroxaban arm.[40] Rivaroxaban increased gastrointestinal bleeding when compared to warfarin.[322]
In the EINSTEIN DVT study, subjects with acute DVT received either warfarin/enoxaparin or rivaroxaban. There was no significant difference in major bleeding between the groups.[45] For PE, the EINSTEIN PE study was conducted identically. There were 26 major bleeding events (1.1%) in the rivaroxaban arm compared to 52 events (2.2%) in the warfarin/enoxaparin arm. Gastrointestinal bleeding rates were not reported.[44] The RECORD series studied rivaroxaban versus enoxaparin for VTE prophylaxis in patients with hip or knee arthroplasty. There was no statistical difference in major bleeding between groups.[323][324][325]
In the ENGAGE AF-TIMI 48 study, where nonvalvular atrial fibrillation patients received either warfarin or edoxaban, major bleeding was less frequent with edoxaban (3.1% versus 3.7%). The most common site of major bleeding was the gastrointestinal tract (including lower, upper, and rectal bleeding). Gastrointestinal bleeding occurred in 205 patients (1.78%) on edoxaban compared to 150 patients (1.27%) on warfarin.[43] In the Hokusai VTE study, major bleeding did not differ between edoxaban and standard anticoagulation, but edoxaban was superior for the composite of major or clinically relevant nonmajor bleeding (8.5% versus 10.3%). Edoxaban was associated with a higher rate of gastrointestinal bleeding compared to warfarin (4.2% versus 3.6%).[41]
Consult local drug formulary for full prescribing information and dose.
Pharmacology: combines with antithrombin to indirectly inactivate factor Xa and thrombin; prevents both fibrin formation and thrombin-induced platelet activation, as well as activation of factors V and VIII.[95][230][231]
Indications: treatment of acute venous thromboembolism (VTE); prevention of VTE in at-risk hospitalized patients; prevention of thromboembolic events in atrial fibrillation; treatment of acute and chronic consumptive coagulopathies (disseminated intravascular coagulation); prevention of thrombosis in arterial and cardiac surgery; peripheral arterial embolism; prevention of thrombosis in dialysis procedures.
Contraindications: severe thrombocytopenia (including heparin-induced thrombocytopenia); when appropriate monitoring cannot be performed (treatment doses); active bleeding (unless bleed is due to disseminated intravascular coagulation).
Cautions: hepatic and renal impairment (no dose adjustment required, but these conditions may increase risk of bleeding).
Drug interactions: antiplatelet agents increase risk of bleeding.
Adverse effects: bleeding; thrombocytopenia; abnormal liver function tests; hypersensitivity; local reactions.
Monitoring: platelets; activated partial thromboplastin time.
Consult local drug formulary for full prescribing information and dose.
Pharmacology: indirectly inhibit factors Xa and IIa by binding to antithrombin; inhibit higher proportion of factor Xa than factor IIa compared to heparin. This drug class includes enoxaparin, dalteparin, and tinzaparin. Tinzaparin is not available in the US.[95][237][238][326][327][328]
Indications: prophylaxis of venous thromboembolism (VTE) in surgical inpatients (e.g., abdominal, hip, knee surgery) and at-risk hospitalized patients; prophylaxis of ischemic events in unstable angina and non-Q-wave myocardial infarction; treatment of acute VTE (enoxaparin); acute treatment of ST-elevation myocardial infarction (enoxaparin); extended treatment of VTE in cancer patients (dalteparin).
Contraindications: active bleeding; thrombocytopenia with a positive test for antiplatelet antibody; history of heparin-induced thrombocytopenia; hypersensitivity to heparin or pork products.
Cautions: recent hemorrhage; diabetic retinopathy; recent gastrointestinal bleed; uncontrolled hypertension; predisposition to bleeding; risk of spinal or epidural hematoma with spinal or epidural anesthesia; pregnant women with mechanical heart valves; renal impairment (dose adjustment required); hepatic impairment (use with caution).
Drug interactions: antiplatelet agents increase risk of bleeding.
Adverse effects: bleeding; thrombocytopenia (less likely to cause heparin-induced thrombocytopenia compared to unfractionated heparin); anemia; abnormal liver function tests; diarrhea; nausea.
Monitoring: anti-Xa activity is rarely required; platelets.
Consult local drug formulary for full prescribing information and dose.
Pharmacology: inhibit factor Xa indirectly by binding to antithrombin; no effect on factor IIa or platelet function. Fondaparinux is currently the only drug available in this class.[201]
Indications: prevention of venous thromboembolism (VTE) in hip, knee, or abdominal surgery; treatment of acute VTE.
Contraindications: creatinine clearance <30 mL/min; active bleeding; acute bacterial endocarditis; positive test for antiplatelet antibody in presence of fondaparinux; body weight <50 kg (VTE prevention).
Cautions: older people (increased risk of hemorrhage); creatinine clearance 30-50 mL/min; should not be used as sole anticoagulant during percutaneous coronary intervention (risk of catheter thrombosis).
Drug interactions: no known significant interactions; drugs that increase risk of hemorrhage should be discontinued prior to use.
Adverse effects: bleeding is the most common adverse effect (see below).
Monitoring: platelets.
Fondaparinux has been compared to enoxaparin for the prevention of VTE in orthopedic surgery in various trials. In addition to elective joint replacement, fondaparinux was also compared to enoxaparin in one randomized controlled trial in patients with hip fracture, and was found to reduce the risk of VTE without an increase in major bleeding.[329] One study in patients undergoing knee arthroplasty found that fondaparinux was associated with a statistically significant increased risk of bleeding compared to enoxaparin (2.1% versus 0.2%); however, the risk was found to be similar in other trials.[330] The risk of major bleeding was also assessed in abdominal surgery trials. In one study, major bleeding was more likely in patients treated with fondaparinux (3.4%) compared to patients treated with dalteparin (2.4%).[331] No difference in bleeding risk was found between fondaparinux and either enoxaparin or unfractionated heparin in patients treated for acute VTE.[332][333]
Consult local drug formulary for full prescribing information and dose.
Pharmacology: low molecular weight heparinoids that catalyze inactivation of factor Xa and also indirectly enhance inactivation of factor IIa; lower cross-reactivity with heparin-associated antibodies in patients with heparin-induced thrombocytopenia (HIT) compared to low molecular weight heparin. Danaparoid is currently the only drug available in this class, although it is not available in the US.[236][334]
Indications: HIT; deep vein thrombosis (DVT) prophylaxis (prior history of HIT); DVT prophylaxis following orthopedic or major abdominal or thoracic surgery; nonhemorrhagic stroke.
Contraindications: bacterial endocarditis; history of major blood clotting disorder; active bleeding; severe untreated hypertension; history of thrombocytopenia with prior use; brain, spine, eye, or ear surgery.
Cautions: gastrointestinal ulcers; renal impairment.
Drug interactions: no known significant interactions; drugs that increase risk of hemorrhage should be discontinued prior to use.
Adverse effects: bleeding is the most common adverse effect (see below); injection site hematoma (5%).
Danaparoid was compared to warfarin for DVT prophylaxis in patients following hip fracture surgery. DVT was found in 7% of patients who were treated with danaparoid and in 21% of patients treated with warfarin. Major bleeding complications did not differ between the two groups.[335]
Consult local drug formulary for full prescribing information and dose.
Pharmacology: bivalent, direct thrombin inhibitors that reversibly and directly bind to both the active enzyme site and the fibrin binding site (unlike argatroban and dabigatran that bind solely to active enzyme site); inhibit both free and clot-bound thrombin. This drug class includes bivalirudin and desirudin.[93][336][337]
Indications: patients with unstable angina undergoing percutaneous transluminal coronary angioplasty (bivalirudin); patients undergoing percutaneous coronary intervention (PCI) with concomitant use of glycoprotein IIb/IIIa inhibitor (bivalirudin); patients undergoing PCI with, or at risk for, heparin-induced thrombocytopenia (bivalirudin); prevention of venous thromboembolism (VTE) in hip surgery (desirudin).
Contraindications: active major bleeding.
Cautions: increased risk of acute stent thrombosis in patients undergoing PCI after ST-elevation myocardial infarction (compared to heparin); renal impairment (dose reduction).
Drug interactions: warfarin (prolongs INR); increased risk of bleeding when given with other anticoagulants, thrombolytics, and antiplatelet agents.
Adverse effects: bleeding (see below); anaphylaxis (some patients develop antibodies and experience anaphylaxis on re-exposure, although this is rare).
In the REPLACE-2 trial, major bleeding occurred less often when bivalirudin was combined with a glycoprotein IIa/IIIb rather than heparin (2.4% versus 4.1%) in patients with acute coronary syndrome undergoing PCI.[338] A similar finding was noted in patients undergoing coronary angioplasty for unstable or postinfarction angina.[339] In patients undergoing VTE prophylaxis following total hip replacement, there was no difference in bleeding risk between desirudin and enoxaparin.[340]
Consult local drug formulary for full prescribing information and dose.
Pharmacology: direct thrombin inhibitor which unlike heparin, low molecular weight heparin, fondaparinux, and danaparoid does not require antithrombin for its activity.[92]
Indications: prophylaxis and treatment of thrombosis in patients with heparin-induced thrombocytopenia (HIT); patients undergoing percutaneous coronary intervention with, or at risk for HIT.
Contraindications: active, clinically significant bleeding.
Cautions: severe uncontrolled hypertension; spinal anesthesia; lumbar puncture; major surgery; gastrointestinal lesions; congenital bleeding disorders; hepatic impairment.
Drug interactions: heparin (should be stopped before argatroban started); warfarin (prolonged prothrombin time/INR); increased risk of bleeding when given with other anticoagulants, thrombolytics, and antiplatelet agents.
Adverse effects: bleeding (see below).
In clinical trials of patients with HIT with or without thrombosis, argatroban was compared to local treatment protocols (usually a vitamin K antagonist-based regimen). Rates of major bleeding were 3.1% for argatroban and 8.2% for control in HIT patients without thrombosis, and 11.1% for argatroban and 2.2% for control in HIT patients with thrombosis.[341] In a study of patients with coronary heart disease who were undergoing elective percutaneous coronary intervention, there was no statistical difference in bleeding between patients treated with argatroban or heparin.[342]
Consult local drug formulary for full prescribing information and dose.
For all anticoagulants, patients should be educated to monitor themselves for signs and symptoms of bleeding, as well as for signs and symptoms of new thrombosis. Patients should also be educated about when to seek medical attention, such as for significant head or abdominal trauma, uncontrolled bleeding from an injury, unremitting epistaxis, hemoptysis, hematochezia, hematuria, severe and unusual headaches, or changes in mental status.
Patients should also be instructed to notify their anticoagulation provider of any planned surgeries or procedures, with sufficient advance notice, to allow guidance on periprocedural anticoagulation management. Patients should be counseled not to start new prescription or over-the-counter medications, herbal medicines, or supplements without consulting their anticoagulant provider. They should also notify all providers that they are on an anticoagulant, particularly with warfarin, so this can be taken into consideration when prescribing other medications.
If patients are selected to perform self-monitoring, they should receive comprehensive training on the use of the home monitor and be able to demonstrate proper technique in performing the INR measurement. In addition to these skills, patients who will perform self-management should receive formalized training on the principles of warfarin dose adjustment; they should also receive a protocol detailing situations in which direct communication to the responsible healthcare provider is required, and be able to demonstrate these skills on a formalized assessment. A significant proportion of patients may not be able to acquire the skills necessary for self-management.[343]
There are no foods that have so far been identified to interact with direct oral anticoagulants; however, rivaroxaban should be taken with a meal to facilitate adequate absorption. For warfarin, a patient’s INR level can be affected by changes in vitamin K intake, overall caloric intake, and ingestion of other fat-soluble vitamins.[344] Decreased vitamin K intake can increase INR, while increased vitamin K intake can decrease INR. Warfarin patients should not be counseled to avoid vitamin K-containing foods but to maintain a consistent diet. Alcohol can increase INR for patients on warfarin, and can increase the bleeding risk for all anticoagulants. Patients should be educated to abstain from alcohol or limit alcohol intake to no more than 1-2 drinks on an occasional basis.[345]
Educating patients regarding the importance of venous thromboembolism prevention while hospitalized results in fewer refused doses of prophylactic anticoagulants.[346]
The National Blood Clot Alliance has a free guide for patient education and information resources regarding thrombosis and anticoagulants.
National Blood Clot Alliance: Stop The Clot® Opens in new window
The American Heart Association offers a set of materials for patients with atrial fibrillation who are taking anticoagulants.
American Heart Association: a patient's guide to taking warfarin Opens in new window
American Society of Hematology
American Society of Hematology 2021 Guidelines for Management of Venous Thromboembolism: Prevention and Treatment in Patients with Cancer[26]
American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism[111]
American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients[347]
American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients[348]
American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy[349]
American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy[139]
American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia[30]
American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism[350]
ASH guidelines on use of anticoagulation in patients with COVID-19[351]
See Coronavirus disease 2019 (COVID-19).
American Society of Hematology 2023 guidelines for management of venous thromboembolism: thrombophilia testing[123]
American College of Chest Physicians
2018 Antithrombotic therapy for atrial fibrillation[352]
2021 Antithrombotic therapy for VTE disease[32]
2012 Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-based clinical practice guidelines[80]
2022 Perioperative management of anticoagulants[18]
Anticoagulation forum
2016 Management of venous thromboembolism: clinical guidance from the Anticoagulation Forum[353]
2022 Thromboembolic prevention and anticoagulant therapy during the COVID-19 pandemic: updated clinical guidance from the Anticoagulation Forum[23]
American College of Cardiology
2020 ACC expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease[19]
2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants[354]
American Society of Clinical Oncology
Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update[29]
European Expert Consensus
European expert consensus recommendations on the primary care use of direct oral anticoagulants in patients with venous thromboembolism.[355]
European Society of Cardiology
2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS)[356]
European Society for Medical Oncology
Venous thromboembolism in cancer patients: ESMO clinical practice guideline[28]
American College of Gastroenterology and Canadian Association of Gastroenterology
2022 Management of anticoagulants and antiplatelets during acute gastrointestinal bleeding and the periendoscopic period[17]
Society of Interventional Radiology
Consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part I: review of anticoagulation agents and clinical considerations[159]
Consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part II: recommendations[160]
Use of this content is subject to our disclaimer