The evaluation of patients with suspected myocarditis begins with a complete history and physical examination. If myocarditis is still suspected, then further work-up with a 12-lead ECG, chest x-ray, laboratory evaluation of cardiac biomarkers, and two-dimensional echo should be performed.[16]Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Nov 30;144(22):e368-454.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001029
http://www.ncbi.nlm.nih.gov/pubmed/34709879?tool=bestpractice.com
[21]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[57]Expert Panel on Cardiac Imaging, Rajiah P, Kirsch J, Bolen MA, et al. ACR Appropriateness Criteria® Nonischemic myocardial disease with clinical manifestations (ischemic cardiomyopathy already excluded). J Am Coll Radiol. 2021 May;18(5S):S83-105.
https://www.jacr.org/article/S1546-1440(21)00115-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33651982?tool=bestpractice.com
Recommendations on which patients should undergo endomyocardial biopsy (EMB) vary and local guidelines should be consulted.[5]Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2013 Sep;34(33):2636-48.
https://academic.oup.com/eurheartj/article/34/33/2636/408735
http://www.ncbi.nlm.nih.gov/pubmed/23824828?tool=bestpractice.com
[58]Bozkurt B, Colvin M, Cook J, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016 Nov 3;134(23):e579-646.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000455
http://www.ncbi.nlm.nih.gov/pubmed/27832612?tool=bestpractice.com
[59]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[60]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[61]Seferović PM, Tsutsui H, McNamara DM, et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society position statement on endomyocardial biopsy. Eur J Heart Fail. 2021 Jun;23(6):854-71.
https://onlinelibrary.wiley.com/doi/10.1002/ejhf.2190
http://www.ncbi.nlm.nih.gov/pubmed/34010472?tool=bestpractice.com
Contrast-enhanced cardiac magnetic resonance imaging (MRI) has been shown to assist in the diagnosis of myocarditis by providing detailed tissue characterisation.[16]Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Nov 30;144(22):e368-454.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001029
http://www.ncbi.nlm.nih.gov/pubmed/34709879?tool=bestpractice.com
[21]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[57]Expert Panel on Cardiac Imaging, Rajiah P, Kirsch J, Bolen MA, et al. ACR Appropriateness Criteria® Nonischemic myocardial disease with clinical manifestations (ischemic cardiomyopathy already excluded). J Am Coll Radiol. 2021 May;18(5S):S83-105.
https://www.jacr.org/article/S1546-1440(21)00115-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33651982?tool=bestpractice.com
[62]Friedrich MG, Strohm O, Schulz-Menger, et al. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998 May 12;97(18):1802-9.
https://www.ahajournals.org/doi/full/10.1161/01.cir.97.18.1802
http://www.ncbi.nlm.nih.gov/pubmed/9603535?tool=bestpractice.com
Positron emission tomography-computed tomography (PET-CT), especially 18F-fluorodeoxyglucose (FDG) PET-CT, can also be of value.[21]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
The approach may be modified for children with suspected myocarditis. The 2021 American Heart Association (AHA) statement on paediatric myocarditis lays out a new paradigm for myocarditis based on diagnostic strata and also provides guidance on approach.[15]Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021 Aug 10;144(6):e123-35.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001001
http://www.ncbi.nlm.nih.gov/pubmed/34229446?tool=bestpractice.com
Cardiac MRI is the preferred diagnostic modality in children because it is non-invasive, but EMB retains specific indications.[15]Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021 Aug 10;144(6):e123-35.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001001
http://www.ncbi.nlm.nih.gov/pubmed/34229446?tool=bestpractice.com
The American College of Cardiology describes the spectrum of myocarditis in 4 stages, mirroring the adult heart failure paradigm: stage A (at risk), stage B (asymptomatic with structural heart disease), stage C (symptomatic myocarditis), and stage D (advanced/refractory myocarditis).[56]Writing Committee, Drazner MH, Bozkurt B, et al. 2024 ACC Expert Consensus Decision Pathway on strategies and criteria for the diagnosis and management of myocarditis: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2025 Feb 4;85(4):391-431.
https://www.jacc.org/doi/10.1016/j.jacc.2024.10.080
http://www.ncbi.nlm.nih.gov/pubmed/39665703?tool=bestpractice.com
Although this classification is based on adult studies, the ACC suggests that the approach can be adapted for paediatric populations. See Criteria.
History
Patients presenting with myocarditis are usually aged <50 years. A history of viral prodrome 2-3 weeks prior to the onset of myocarditis is commonly present. Symptoms may include chest pain, dyspnoea, orthopnoea, syncope, fatigue, and palpitations.[16]Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Nov 30;144(22):e368-454.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001029
http://www.ncbi.nlm.nih.gov/pubmed/34709879?tool=bestpractice.com
In children, a viral prodrome will be the most common history, followed by history suggestive of arrhythmias or syncope.[15]Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021 Aug 10;144(6):e123-35.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001001
http://www.ncbi.nlm.nih.gov/pubmed/34229446?tool=bestpractice.com
The common symptoms include fever, fatigue, respiratory symptoms, abdominal pain, emesis, and lethargy.[15]Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021 Aug 10;144(6):e123-35.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001001
http://www.ncbi.nlm.nih.gov/pubmed/34229446?tool=bestpractice.com
The patient's medical history may be significant for autoimmune disease such as collagen-vascular diseases as well as inflammatory bowel disease, diabetes mellitus, sarcoidosis, thyrotoxicosis, granulomatosis with polyangiitis (formerly known as Wegener's granulomatosis), or Loeffler's syndrome. In addition, a history of travel to endemic areas where causative organisms are found may suggest a specific infectious aetiology.
A thorough drug history along with other possible exposures to toxic substances or cocaine should be obtained to exclude any drug or toxic aetiologies. These include anthracyclines (e.g., doxorubicin, daunorubicin, epirubicin, idarubicin), fluoropyrimidines (e.g., fluorouracil, capecitabine), immunotherapies (e.g., ipilimumab, tremelimumab, nivolumab, pembrolizumab, cemiplimab, atezolizumab, avelumab, durvalumab, and trastuzumab), arsenic, zidovudine, carbon monoxide, ethanol, interleukin-2, cocaine, amphetamine, smallpox or mpox vaccine, SARS-CoV-2 (COVID-19) mRNA vaccine (may occur with other types of COVID-19 vaccines), catecholamines (e.g., adrenaline, noradrenaline, dopamine), cyclophosphamide, heavy metals (copper, iron, lead), radiation, antibiotics (penicillins, cephalosporins, sulfonamides), amphotericin B, thiazide diuretics, anticonvulsants (carbamazepine, phenytoin, phenobarbital), digoxin, lithium, amitriptyline, clozapine, snake venom, bee venom, black widow spider venom, scorpion venom, and wasp venom.[30]Truong DT, Dionne A, Muniz JC, et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation. 2022 Feb;145(5):345-56.
https://www.doi.org/10.1161/CIRCULATIONAHA.121.056583
http://www.ncbi.nlm.nih.gov/pubmed/34865500?tool=bestpractice.com
[31]Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022 Jan 25;327(4):331-40.
https://www.doi.org/10.1001/jama.2021.24110
http://www.ncbi.nlm.nih.gov/pubmed/35076665?tool=bestpractice.com
[32]Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022 Nov 1;43(41):4229-361.
https://academic.oup.com/eurheartj/article/43/41/4229/6673995?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017568?tool=bestpractice.com
[52]Halsell JS, Riddle JR, Atwood JE, et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA. 2003 Jun 25;289(24):3283-9.
http://jama.jamanetwork.com/article.aspx?articleid=196808
http://www.ncbi.nlm.nih.gov/pubmed/12824210?tool=bestpractice.com
[53]Burke AP, Saenger J, Mullick R, et al. Hypersensitivity myocarditis. Arch Pathol Lab Med. 1991 Aug;115(8):764-9.
http://www.ncbi.nlm.nih.gov/pubmed/1863186?tool=bestpractice.com
[54]Ansari A, Maron BJ, Berntson DG. Drug-induced toxic myocarditis. Tex Heart Inst J. 2003;30(1):76-9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC152844
http://www.ncbi.nlm.nih.gov/pubmed/12638679?tool=bestpractice.com
Physical examination
A thorough examination for both the cardiorespiratory findings and other general findings that may point to possible aetiology should be performed. Findings can include rales, elevated neck veins, S3 gallop, S3 and S4 summation gallop, pericardial friction rub, peripheral hypoperfusion, sinus tachycardia, atrial and ventricular arrhythmias, hypotension, altered sensorium, hepatomegaly, and lymphadenopathy. Resting tachycardia is an especially important sign in adolescents where the symptoms can be masked significantly in both acute and chronic myocarditis.
12-lead ECG
A 12-lead ECG should be ordered immediately on presentation in anyone with chest pain or cardiac symptoms.[15]Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021 Aug 10;144(6):e123-35.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001001
http://www.ncbi.nlm.nih.gov/pubmed/34229446?tool=bestpractice.com
[16]Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Nov 30;144(22):e368-454.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001029
http://www.ncbi.nlm.nih.gov/pubmed/34709879?tool=bestpractice.com
In myocarditis it most commonly displays non-specific ST-segment and T-wave abnormalities; however, ST-segment elevation and depression frequently occur. Children can present with atrial and ventricular arrhythmias and such a presentation can be a marker of poor outcomes.[63]Ichikawa R, Sumitomo N, Komori A, et al. The follow-up evaluation of electrocardiogram and arrhythmias in children with fulminant myocarditis. Circ J. 2011;75(4):932-8.
https://www.doi.org/10.1253/circj.cj-10-0918
http://www.ncbi.nlm.nih.gov/pubmed/21343655?tool=bestpractice.com
A component of pericarditis is common in children and can be evident as non-specific ST-segment changes in all of the precordial leads.
Laboratory evaluation
Cardiac biomarkers should be ordered immediately in any patient with suspected myocarditis. Creatine kinase and creatine kinase-MB levels are often mildly elevated. Troponin (I or T) levels have shown to be a more reliable indicator of myocardial damage, including in children.[15]Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021 Aug 10;144(6):e123-35.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001001
http://www.ncbi.nlm.nih.gov/pubmed/34229446?tool=bestpractice.com
[16]Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Nov 30;144(22):e368-454.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001029
http://www.ncbi.nlm.nih.gov/pubmed/34709879?tool=bestpractice.com
However, it should be noted that some patients with myocarditis do not have elevated high-sensitivity cardiac troponin levels.[56]Writing Committee, Drazner MH, Bozkurt B, et al. 2024 ACC Expert Consensus Decision Pathway on strategies and criteria for the diagnosis and management of myocarditis: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2025 Feb 4;85(4):391-431.
https://www.jacc.org/doi/10.1016/j.jacc.2024.10.080
http://www.ncbi.nlm.nih.gov/pubmed/39665703?tool=bestpractice.com
Serum B-type natriuretic peptide may be helpful in distinguishing primary cardiac from primary pulmonary aetiologies of dyspnoea when the physical examination and initial work-up is unclear or non-specific. In myocarditis it is elevated in response to ventricular distention such as occurs in congestive heart failure.[59]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Chest x-ray (CXR)
Should be ordered in all patients with suspected myocarditis.[64]Schulz-Menger J, Collini V, Gröschel J, et al. 2025 ESC guidelines for the management of myocarditis and pericarditis. Eur Heart J. 2025 Oct 22;46(40):3952-4041.
https://academic.oup.com/eurheartj/article/46/40/3952/8234483?login=false
http://www.ncbi.nlm.nih.gov/pubmed/40878297?tool=bestpractice.com
As a first-line investigation, CXR helps rule out pulmonary oedema and provides an initial assessment of cardiac silhouette and pulmonary vasculature.[16]Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Nov 30;144(22):e368-454.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001029
http://www.ncbi.nlm.nih.gov/pubmed/34709879?tool=bestpractice.com
In the context of acute heart failure due to myocarditis, CXR may reveal pulmonary congestion. Cardiomegaly can suggest associated myopericarditis with pericardial effusion or chronic myocarditis evolving into dilated cardiomyopathy.
Two-dimensional echocardiogram
This should be ordered in every patient suspected of having myocarditis.[15]Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021 Aug 10;144(6):e123-35.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001001
http://www.ncbi.nlm.nih.gov/pubmed/34229446?tool=bestpractice.com
[16]Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Nov 30;144(22):e368-454.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001029
http://www.ncbi.nlm.nih.gov/pubmed/34709879?tool=bestpractice.com
[57]Expert Panel on Cardiac Imaging, Rajiah P, Kirsch J, Bolen MA, et al. ACR Appropriateness Criteria® Nonischemic myocardial disease with clinical manifestations (ischemic cardiomyopathy already excluded). J Am Coll Radiol. 2021 May;18(5S):S83-105.
https://www.jacr.org/article/S1546-1440(21)00115-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33651982?tool=bestpractice.com
Myocarditis can cause global and regional left ventricular motion abnormalities, systolic and diastolic dysfunction, and dilatation.[Figure caption and citation for the preceding image starts]: Apical 4-chamber transthoracic echocardiogram in a patient with myocarditis. The right ventricle is dilated with hypokinesis. Triscupid regurgitation is present with a reduced continuous wave Doppler gradient indicating right ventricular failureFrom: Rasmussen TB, Dalager S, Andersen NH, et al. BMJ Case Reports 2009; doi:10.1136/bcr.09.2008.0997 [Citation ends]. [Figure caption and citation for the preceding image starts]: Apical 4-chamber echocardiogram in a patient presenting with myocarditis showing a slightly dilated left ventricle with spontaneous ultrasonic contrast indicating severely impaired left ventricular systolic functionFrom: Rasmussen TB, Dalager S, Andersen NH, et al. BMJ Case Reports 2009; doi:10.1136/bcr.09.2008.0997 [Citation ends].
[Figure caption and citation for the preceding image starts]: Apical 4-chamber echocardiogram in a patient presenting with myocarditis showing a slightly dilated left ventricle with spontaneous ultrasonic contrast indicating severely impaired left ventricular systolic functionFrom: Rasmussen TB, Dalager S, Andersen NH, et al. BMJ Case Reports 2009; doi:10.1136/bcr.09.2008.0997 [Citation ends].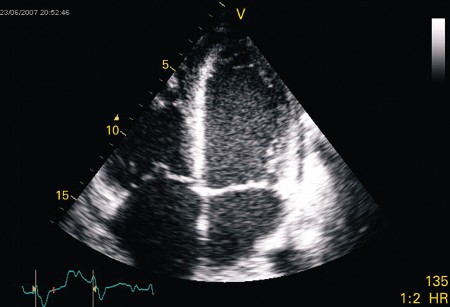 Special attention should be paid to evaluation for clots which can change therapies and risk profile. Development of intracardiac thrombosis can occur due to a prothrombotic, proinflammatory state as well as stagnant blood in the dysfunctional heart.[65]Skouri HN, Dec GW, Friedrich MG, et al. Noninvasive imaging in myocarditis. J Am Coll Cardiol. 2006 Nov 21;48(10):2085-93.
https://www.doi.org/10.1016/j.jacc.2006.08.017
http://www.ncbi.nlm.nih.gov/pubmed/17112998?tool=bestpractice.com
Special attention should be paid to evaluation for clots which can change therapies and risk profile. Development of intracardiac thrombosis can occur due to a prothrombotic, proinflammatory state as well as stagnant blood in the dysfunctional heart.[65]Skouri HN, Dec GW, Friedrich MG, et al. Noninvasive imaging in myocarditis. J Am Coll Cardiol. 2006 Nov 21;48(10):2085-93.
https://www.doi.org/10.1016/j.jacc.2006.08.017
http://www.ncbi.nlm.nih.gov/pubmed/17112998?tool=bestpractice.com
Endomyocardial biopsy (EMB)
The potential risks and benefits of EMB should always be carefully assessed, particularly during the acute presentation, as the risk of arrhythmia or ventricular perforation is highest at this time. Recommendations on which patients should undergo EMB vary and local guidelines should be consulted.[5]Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2013 Sep;34(33):2636-48.
https://academic.oup.com/eurheartj/article/34/33/2636/408735
http://www.ncbi.nlm.nih.gov/pubmed/23824828?tool=bestpractice.com
[58]Bozkurt B, Colvin M, Cook J, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016 Nov 3;134(23):e579-646.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000455
http://www.ncbi.nlm.nih.gov/pubmed/27832612?tool=bestpractice.com
[59]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[60]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[61]Seferović PM, Tsutsui H, McNamara DM, et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society position statement on endomyocardial biopsy. Eur J Heart Fail. 2021 Jun;23(6):854-71.
https://onlinelibrary.wiley.com/doi/10.1002/ejhf.2190
http://www.ncbi.nlm.nih.gov/pubmed/34010472?tool=bestpractice.com
[66]Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007 Nov 6;116(19):2216-33.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.107.186093
http://www.ncbi.nlm.nih.gov/pubmed/17959655?tool=bestpractice.com
[67]Ammirati E, Frigerio M, Adler ED, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020 Nov;13(11):e007405.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7673642
http://www.ncbi.nlm.nih.gov/pubmed/33176455?tool=bestpractice.com
The ACC recommends EMB in adults with suspected:[56]Writing Committee, Drazner MH, Bozkurt B, et al. 2024 ACC Expert Consensus Decision Pathway on strategies and criteria for the diagnosis and management of myocarditis: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2025 Feb 4;85(4):391-431.
https://www.jacc.org/doi/10.1016/j.jacc.2024.10.080
http://www.ncbi.nlm.nih.gov/pubmed/39665703?tool=bestpractice.com
Asymptomatic (stage B) myocarditis in the setting of immune checkpoint inhibitor therapy
Symptomatic (stage C) myocarditis with left ventricular dysfunction, symptomatic heart failure, arrhythmia, peripheral eosinophilia, or when the diagnosis is uncertain and cardiac MRI cannot be obtained
Most patients with advanced (stage D) myocarditis
The ACC recommends against EMB for low-risk adults with stage C myocarditis (i.e., symptomatic myocarditis with normal left ventricular ejection fraction, haemodynamic and electrical stability, and no or minimal late gadolinium enhancement) and adults with stage B myocarditis that is not due to immune checkpoint inhibitor therapy.[56]Writing Committee, Drazner MH, Bozkurt B, et al. 2024 ACC Expert Consensus Decision Pathway on strategies and criteria for the diagnosis and management of myocarditis: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2025 Feb 4;85(4):391-431.
https://www.jacc.org/doi/10.1016/j.jacc.2024.10.080
http://www.ncbi.nlm.nih.gov/pubmed/39665703?tool=bestpractice.com
A statement on dilated cardiomyopathies from the AHA recommends biopsy for adults 'with clinically suspected unexplained acute myocarditis who require inotropic support or mechanical circulatory support and those with Mobitz type 2 second-degree or higher heart block, sustained or symptomatic ventricular tachycardia, or failure to respond to guideline-based medical management within 1-2 weeks'.[58]Bozkurt B, Colvin M, Cook J, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016 Nov 3;134(23):e579-646.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000455
http://www.ncbi.nlm.nih.gov/pubmed/27832612?tool=bestpractice.com
The 2022 AHA/ACC/Heart Failure Society of America (HFSA) adult heart failure (HF) guidelines state 'Endomyocardial biopsy may be advantageous in patients with heart failure in which a histological diagnosis, such as myocarditis, may influence treatment decisions'.[59]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
A joint statement from Heart Failure Association of European Society of Cardiology (ESC), HFSA, and Japanese HF Society recommends EMB 'in patients with fulminant/acute myocarditis presenting with cardiogenic shock or acute HF and left ventricular (LV) dysfunction, with or without malignant ventricular arrhythmias and/or conduction abnormalities'. They also recommend considering EMB in 'haemodynamically stable patients with clinical symptoms and diagnostic criteria (electrocardiographic abnormalities, elevated troponin levels, and imaging findings) suggestive of myocarditis, in the absence of significant coronary artery disease'.[61]Seferović PM, Tsutsui H, McNamara DM, et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society position statement on endomyocardial biopsy. Eur J Heart Fail. 2021 Jun;23(6):854-71.
https://onlinelibrary.wiley.com/doi/10.1002/ejhf.2190
http://www.ncbi.nlm.nih.gov/pubmed/34010472?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Histological findings in a patient with giant cell myocarditis. A: severe myocardial necrosis and fibrotic replacement of the cardiomyocytes with granulation tissue and fibrosis is present in a section from the anterolateral left ventricular wall; B: a sharp demarcating border between vital and necrotic myocardium is seen, confirmed by additional immunohistochemical staining for myoglobin; C: at the inflammatory border, cells consisting of prominent multi-nucleated giant cells, macrophages, lymphocytes, and eosinophilic granulocytes are seen in close proximity to vital myocardium; D: immunohistochemical staining for complement 4d is positive in all vessels, suggestive of complement cascade activationFrom: Rasmussen TB, Dalager S, Andersen NH, et al. BMJ Case Reports 2009; doi:10.1136/bcr.09.2008.0997 [Citation ends].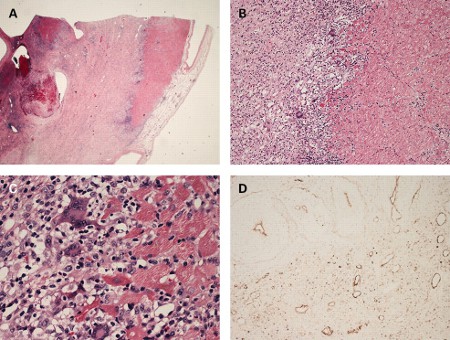
Additionally, the biopsies can be used for viral polymerase chain reaction (PCR) testing to diagnose viral myocarditis with specificity, and to analyse immune-mediated injury and potentially inflammatory markers at a molecular level.[68]Bowles NE, Ni J, Kearney DL, et al. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003 Aug 6;42(3):466-72.
https://www.doi.org/10.1016/s0735-1097(03)00648-x
http://www.ncbi.nlm.nih.gov/pubmed/12906974?tool=bestpractice.com
In children, cardiac MRI has replaced traditional EMB as the preferred diagnostic modality because it is non-invasive.[15]Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021 Aug 10;144(6):e123-35.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001001
http://www.ncbi.nlm.nih.gov/pubmed/34229446?tool=bestpractice.com
However, EMB retains specific indications, particularly in confirming diagnoses such as lymphocytic myocarditis, hypersensitivity myocarditis, and giant cell myocarditis, where histopathological evaluation directly informs clinical management. Immunohistochemistry has greatly increased the ability to assess for inflammatory cells.[15]Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021 Aug 10;144(6):e123-35.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001001
http://www.ncbi.nlm.nih.gov/pubmed/34229446?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Histological findings in a patient with giant cell myocarditis. A: severe myocardial necrosis and fibrotic replacement of the cardiomyocytes with granulation tissue and fibrosis is present in a section from the anterolateral left ventricular wall; B: a sharp demarcating border between vital and necrotic myocardium is seen, confirmed by additional immunohistochemical staining for myoglobin; C: at the inflammatory border, cells consisting of prominent multi-nucleated giant cells, macrophages, lymphocytes, and eosinophilic granulocytes are seen in close proximity to vital myocardium; D: immunohistochemical staining for complement 4d is positive in all vessels, suggestive of complement cascade activationFrom: Rasmussen TB, Dalager S, Andersen NH, et al. BMJ Case Reports 2009; doi:10.1136/bcr.09.2008.0997 [Citation ends].
Cardiac MRI
Cardiac MRI is as an important tool in the assessment of suspected myocarditis, particularly when trying to distinguish acute myocarditis from acute MI.[16]Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Nov 30;144(22):e368-454.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001029
http://www.ncbi.nlm.nih.gov/pubmed/34709879?tool=bestpractice.com
[57]Expert Panel on Cardiac Imaging, Rajiah P, Kirsch J, Bolen MA, et al. ACR Appropriateness Criteria® Nonischemic myocardial disease with clinical manifestations (ischemic cardiomyopathy already excluded). J Am Coll Radiol. 2021 May;18(5S):S83-105.
https://www.jacr.org/article/S1546-1440(21)00115-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33651982?tool=bestpractice.com
[62]Friedrich MG, Strohm O, Schulz-Menger, et al. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998 May 12;97(18):1802-9.
https://www.ahajournals.org/doi/full/10.1161/01.cir.97.18.1802
http://www.ncbi.nlm.nih.gov/pubmed/9603535?tool=bestpractice.com
In some cases, findings from cardiac MRI can also be useful in determining the aetiology of myocarditis.[69]Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006 Oct 10;114(15):1581-90.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.105.606509
http://www.ncbi.nlm.nih.gov/pubmed/17015795?tool=bestpractice.com
Lake Louise consensus criteria were updated in 2018 to propose that presence of both T2 and T1 findings provide strong evidence for myocardial inflammation.[70]Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018 Dec 18;72(24):3158-76.
https://www.sciencedirect.com/science/article/pii/S0735109718388430
http://www.ncbi.nlm.nih.gov/pubmed/30545455?tool=bestpractice.com
In children, the primary purpose of cardiac MRI is to identify myocardial injury, and to differentiate acute myocarditis from non-inflammatory cardiomyopathies.[15]Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021 Aug 10;144(6):e123-35.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001001
http://www.ncbi.nlm.nih.gov/pubmed/34229446?tool=bestpractice.com
Other tests
Coronary angiography: this should be performed when the presenting symptoms and findings are indistinguishable from the acute coronary syndromes.
FDG PET-CT: helps diagnose myocarditis by providing metabolic information of inflammation as increased FDG uptake. FDG PET-CT is useful in chronic myocarditis, where cardiac magnetic resonance does not have the same accuracy as in acute myocarditis.[71]Chen W, Jeudy J. Assessment of myocarditis: cardiac MR, PET/CT, or PET/MR? Curr Cardiol Rep. 2019 Jun 26;21(8):76.
http://www.ncbi.nlm.nih.gov/pubmed/31243587?tool=bestpractice.com
FDG PET-CT is also a valuable diagnostic tool for cardiac sarcoidosis and can facilitate differentiation from giant cell myocarditis.[72]Fraser EJ, Culver A, Lam PH, et al. Giant cell myocarditis vs cardiac sarcoidosis: reconsidering the diagnosis with FDG PET imaging. JACC Case Rep. 2024 Nov 20;29(22):102738.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11646884
http://www.ncbi.nlm.nih.gov/pubmed/39691891?tool=bestpractice.com
In cardiac sarcoidosis presenting with myocarditis, FDG PET-CT typically reveals tracer uptake in the lungs, lymph nodes, and myocardial tissue. In addition, FDG PET-CT can help detect myocarditis in the context of systemic autoimmune diseases, where abnormal tracer accumulation may be seen in the myocardium as well as other involved organs, such as the aortic wall in certain forms of vasculitis.[67]Ammirati E, Frigerio M, Adler ED, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020 Nov;13(11):e007405.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7673642
http://www.ncbi.nlm.nih.gov/pubmed/33176455?tool=bestpractice.com
MRI-guided EMB: this is a promising new technology that seems to significantly increase the sensitivity of EMB in the diagnosis of myocarditis.[73]Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004 Mar 16;109(10):1250-8.
https://www.ahajournals.org/doi/10.1161/01.cir.0000118493.13323.81
http://www.ncbi.nlm.nih.gov/pubmed/14993139?tool=bestpractice.com
This is still in the developmental phase and requires validation in larger studies.
Genetic testing: genetic predisposition is increasingly recognised as a risk factor for myocarditis.[55]Monda E, Limongelli G. Is there a role for genetic testing in patients with myocarditis? Circ Genom Precis Med. 2022 Oct;15(5):e003824.
https://www.ahajournals.org/doi/10.1161/CIRCGEN.122.003824?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/36173695?tool=bestpractice.com
The ACC recommends genetic counselling and testing for all consenting patients, followed by cascade screening of family members if a pathogenic variant is identified to facilitate early detection, surveillance, and management of at-risk relatives.[56]Writing Committee, Drazner MH, Bozkurt B, et al. 2024 ACC Expert Consensus Decision Pathway on strategies and criteria for the diagnosis and management of myocarditis: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2025 Feb 4;85(4):391-431.
https://www.jacc.org/doi/10.1016/j.jacc.2024.10.080
http://www.ncbi.nlm.nih.gov/pubmed/39665703?tool=bestpractice.com
 [Figure caption and citation for the preceding image starts]: Apical 4-chamber echocardiogram in a patient presenting with myocarditis showing a slightly dilated left ventricle with spontaneous ultrasonic contrast indicating severely impaired left ventricular systolic functionFrom: Rasmussen TB, Dalager S, Andersen NH, et al. BMJ Case Reports 2009; doi:10.1136/bcr.09.2008.0997 [Citation ends].
[Figure caption and citation for the preceding image starts]: Apical 4-chamber echocardiogram in a patient presenting with myocarditis showing a slightly dilated left ventricle with spontaneous ultrasonic contrast indicating severely impaired left ventricular systolic functionFrom: Rasmussen TB, Dalager S, Andersen NH, et al. BMJ Case Reports 2009; doi:10.1136/bcr.09.2008.0997 [Citation ends]. Special attention should be paid to evaluation for clots which can change therapies and risk profile. Development of intracardiac thrombosis can occur due to a prothrombotic, proinflammatory state as well as stagnant blood in the dysfunctional heart.[65]
Special attention should be paid to evaluation for clots which can change therapies and risk profile. Development of intracardiac thrombosis can occur due to a prothrombotic, proinflammatory state as well as stagnant blood in the dysfunctional heart.[65]
