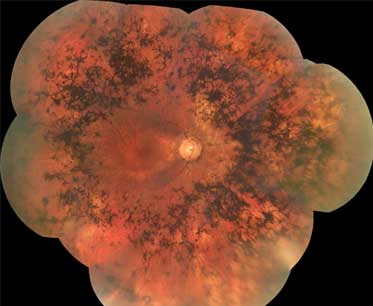
Summary
Definition
History and exam
Key diagnostic factors
- presence of risk factors
- positive family history
- presence of an associated syndrome
- decreased peripheral vision
- night blindness
- impaired dark adaptation
- decreased central acuity
- atrophy of retinal pigment epithelium
- bone spicule pigmentation
Other diagnostic factors
- waxy pale optic nerve
- photopsias
- refractive error
- cataracts
- retinal vascular attenuation
- cystoid macular oedema
- vitreous cells
- glare from bright lights
- abnormal colour vision
- keratoconus
- glaucoma
- optic nerve head drusen
- Coats-like retinopathy
- Leber's congenital amaurosis
Diagnostic investigations
1st investigations to order
- assessment of visual acuity
- anterior segment examination and intraocular pressure measurement
- full field perimetry
- full field electroretinogram (ERG)
Investigations to consider
- elevated final dark-adapted threshold
- optical coherence tomography (OCT)
- genetic testing
- adaptive optics imaging
- wide-field fundus autofluorescence (FAF)
Treatment algorithm
Contributors
Authors
Lesley Everett , MD, PhD, MPhil
Assistant Professor of Ophthalmology
Casey Eye Institute
Oregon Health and Sciences University
Divisions of Ophthalmic Genetics and Retina
Portland
OR
Disclosures
LAE is supported by a Foundation Fighting Blindness Career Development Grant.
Mark E. Pennesi, MD, PhD

Professor
Casey Eye Institute
Oregon Health and Sciences University
Portland
OR
Disclosures
MEP serves on the scientific advisory board and executive committee for the Foundation Fighting Blindness.
Paul Yang, MD, PhD

Associate Professor
Casey Eye Institute
Oregon Health and Sciences University
Portland
OR
Disclosures
PY acted as a consultant for 4D Molecular Therapeutics, AAVantgarde Bio (IDMC), Adverum, Astellas, Beacon Therapeutics, BlueRock Therapeutics, Eluminex Biosciences, Foundation Fighting Blindness (SAB), Janssen (DSMB), MieraGTx (DSMB), Nanoscope Therapeutics (SAB), and TeamedOn.
Acknowledgements
Dr Lesley Everett, Dr Mark E. Pennesi, and Dr Paul Yang would like to gratefully acknowledge Dr Richard G. Weleber and Dr Peter J. Francis, previous contributors to this topic.
Disclosures
RGW has served as a consultant to Novartis, Pfizer, and Wellstat, is a member of the scientific advisory board for Applied Genetic Technologies Corp, and serves on the scientific advisory board for the Foundation Fighting Blindness (the relationship has been reviewed and managed by Oregon Health & Science University). RGW also reports having received grants and personal fees from the Foundation Fighting Blindness and Applied Genetic Technologies Corp, and other support from Sanofi-Fovea, all outside the submitted work. In addition, RGW has a patent (US patent 8,657,446, Method and apparatus for visual field monitoring, also known as Visual Field Monitoring and Analysis, or VFMA, which has not been issued). PJF declares that he has no competing interests.
Peer reviewers
Scott Fraser, MD, FRCS (Ed), FRCOphth
Consultant Ophthalmologist
Sunderland Eye Infirmary
Sunderland
UK
Disclosures
SF declares that he has no competing interests.
Elias Traboulsi, MD
Professor of Ophthalmology
Director
Center for Genetic Eye Diseases
Cole Eye Institute
Cleveland Clinic
Cleveland
OH
Disclosures
ET declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
American Academy of Ophthalmology. Comprehensive adult medical eye evaluation PPP. Nov 2020 [internet publication].Full text
American Academy of Ophthalmology. Guidelines on clinical assessment of patients with inherited retinal degenerations - 2022. Oct 2022 [internet publication].Full text
Robson AG, Frishman LJ, Grigg J, et al. ISCEV Standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol. 2022 Jun;144(3):165-77.Full text Abstract
American Academy of Ophthalmology. Recommendations for genetic testing of inherited eye diseases. February 2014 [internet publication].Full text
Reference articles
A full list of sources referenced in this topic is available here.
Use of this content is subject to our disclaimer