History and exam
Key diagnostic factors
common
presence of risk factors
Strong risk factors include: obesity; pubertal status; Indo-Asian or black ethnicity; positive family history/genetic predisposition.
polyuria
Typically present in patients with a fasting plasma glucose >16.7 mmol/L (>300 mg/dL) and/or haemoglobin A1c (HbA1c) >86 mmol/mol (>10%).
polydipsia
Typically present in patients with a fasting plasma glucose >16.7 mmol/L (>300 mg/dL) and/or HbA1c >86 mmol/mol (>10%).
acanthosis nigricans
Present in 90% to 95% of patients.[66]
A cutaneous manifestation of insulin resistance characterised by velvety, hyper-pigmented skin, most often in the intertriginous areas. [Figure caption and citation for the preceding image starts]: Acanthosis nigricansFrom the collection of Dr Jennifer Miller [Citation ends].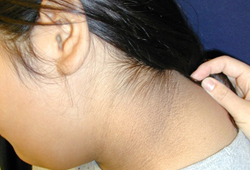 [Figure caption and citation for the preceding image starts]: Acanthosis nigricans in a child with obesityFrom the collection of Dr Jennifer Miller [Citation ends].
[Figure caption and citation for the preceding image starts]: Acanthosis nigricans in a child with obesityFrom the collection of Dr Jennifer Miller [Citation ends]. [Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the folds of the neckFrom the collection of Dr Jennifer Miller [Citation ends].
[Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the folds of the neckFrom the collection of Dr Jennifer Miller [Citation ends].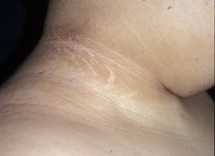 [Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the axillaFrom the collection of Dr Jennifer Miller [Citation ends].
[Figure caption and citation for the preceding image starts]: Acanthosis nigricans in the axillaFrom the collection of Dr Jennifer Miller [Citation ends].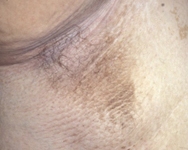
Not specific for type 2 diabetes mellitus, and can also be seen in children with obesity and type 1 diabetes mellitus.
uncommon
nocturia
Due to glucose-induced diuresis.
Other diagnostic factors
common
hypertension
Frequently present at the time of diagnosis.
yeast infections
Most commonly in vaginal and penile areas, or in between skin folds.
skin infections
Cellulitis or abscesses.
urinary tract infections
Cystitis or pyelonephritis.
fatigue
Due to elevated glucose and/or comorbidities.
blurred vision
Due to elevated glucose and/or comorbidities.
uncommon
weight loss
Typically, little or no weight loss, although may be present if marked hyperglycaemia is present.
Risk factors
strong
obesity
UK audit data has found that 90% of children and young people with type 2 diabetes mellitus (T2DM) also have obesity or overweight.[26] Global prevalence of obesity among paediatric patients with T2DM has been reported as 75%.[27]
Visceral fat is more metabolically active than subcutaneous fat and produces adipokines that cause insulin resistance. The amount of visceral fat in adolescents with obesity directly correlates with basal and glucose-stimulated insulin levels and inversely with insulin sensitivity.[44]
genetic predisposition/family history
A 3.5 times greater risk in siblings of affected individuals as compared with the general population.
About 80% to 100% concordance in monozygotic twins.[40]
In the Treatment Options for type 2 Diabetes mellitus (T2DM) in Adolescents and Youth (TODAY) study of American youth with recent-onset T2DM, almost 60% reported at least one parent, full sibling, or half-sibling with diabetes, rising to almost 90% when grandparents were included.[41]
T2DM in children and adolescents, as in adults, is polygenic. While there have been rapid advances in the understanding of the genetics of T2DM in adults, the genetics of T2DM in youth remain largely understudied. In 2021, the Progress in Genetic studies of youth-onset diabetes (ProDiGY) consortium published the first genome-wide association study on youth-onset T2DM, identifying seven crucial loci in the genome, including rs7903146 in TCF7L2, rs72982988 near MC4R, rs200893788 in CDC123, rs2237892 in KCNQ1, rs937589119 in IGF2BP2, rs113748381 in SLC16A11, and rs2604566 in CPEB2, which may play a significant role in early detection of the disease in the future.[42]
high-risk ethnic background
The majority of childhood-onset type 2 diabetes mellitus (T2DM) occurs in children from a high-risk racial/ethnic background.[13][14][15] In the US, between 1990 and 1998, the number of American-Indian and Alaskan native children diagnosed with T2DM increased by 71%.[14] In the US, the highest prevalence of T2DM per 1000 youth in 2017 was observed among black or African-American youth at 1.80, followed by 1.63 in American-Indian youth, 1.03 among youth of Hispanic origin, 0.59 among Asian/Pacific Islander youth, and 0.20 among non-Hispanic white youth.[16] While type 1 diabetes mellitus (T1DM) remains the predominant form of diabetes in children and adolescents, prevalence of T2DM is now higher than that of T1DM among American-Indian youth.[16] Ethnic differences in insulin sensitivity are indicated by greater insulin responses to oral glucose in African-American children and adolescents compared with European-American children, adjusted for weight, age, and pubertal stage.[39]
puberty
The average age of diagnosis of type 2 diabetes mellitus (T2DM) is 14 years and most children are diagnosed between ages 10 and 19 years.[36][37] During puberty, there is a surge in growth hormone and insulin-like growth factor-I (IGF-1), which increases insulin resistance. Almost always, this insulin resistance is transient and any hyperglycaemia reverts to normal after puberty.[37] In addition, an increase in sex hormones during puberty, especially androstenedione, increases the acute insulin response, which is an independent predictor of T2DM.[37] Puberty may also precipitate beta-cell failure in the presence of pre-existing insulin resistance; therefore, young people with obesity, who tend to be more insulin-resistant than those without obesity at the onset of puberty, are more likely to progress to T2DM at this point.[38] Data suggest that young people with obesity do not recover insulin sensitivity at the end of puberty, which may have a negative impact on beta-cell function during this time.[38]
Puberty also heralds a period of change in other cardiometabolic risk factors, such as lipid profile, blood pressure, and adipokines. This has significant implications for young people with obesity: there is evidence that puberty is one of the greatest risk factors for transition from metabolically healthy to unhealthy obesity.[38]
female sex
diabetic in-utero environment
Children exposed to a diabetic intrauterine environment have a 3.7 times increased risk as compared with siblings born before the mother became diabetic.[30]
weak
small for gestation age
In-utero programming as a result of antenatal growth restraint and limited nutrients in utero limits beta-cell capacity and induces insulin resistance in peripheral tissues.[31]
rapid growth in infancy
Rapid weight gain between birth and age 2 years, particularly in low-birth-weight babies, is associated with increased central adiposity and insulin resistance.[32][33] One systematic review found that children experiencing rapid weight gain during the first 2 years of life had a 3.66 times greater chance of developing overweight or obesity later in life than those who did not experience rapid weight gain.[50] The mechanism through which rapid weight gain programmes subsequent adiposity remains unclear.
bottle feeding
Breastfeeding reduces the odds ratio for childhood obesity by approximately 20% as compared with formula feeding.[35] Formula feeding is more likely to be associated with overfeeding. Breastfeeding provides a more appropriate caloric intake at a critical stage in development.
high protein intake in infancy
polycystic ovaries
Associated with insulin resistance and hyperinsulinism, which predisposes to type 2 diabetes mellitus.[52]
intra-myocellular lipid content
fat deposition in the liver
Elevations in alanine aminotransferase are associated with a decline in hepatic insulin sensitivity and the development of type 2 diabetes mellitus.[47]
learning disability
Adjusted analyses show that if factors such as age, sex, ethnic background, and financial year are taken into account, people with a learning disability have an incidence rate for type 2 diabetes mellitus (T2DM) that is 1.65 times (approximately 65%) higher than the rate in the general population.[53] The increase in rates of T2DM in people with a learning disability is shifted 10-15 years earlier than the general population, and for young people with a learning disability (aged 15-24 years), the rate of T2DM is approximately 16 times higher than for their peers without a learning disability.[53] The presence of certain genetic syndromes such as Prader-Willi syndrome or Down syndrome, having low levels of physical activity and poor diet, and being on an antipsychotic may contribute to the higher risk of developing T2DM in individuals with a learning disability.[53]
People with a learning disability may experience a delay in diagnosis which can lead to more severe health complications.[54] UK national diabetes audit data suggest that individuals with a learning disability and T2DM may be less likely to receive appropriate diabetes care, including regular check-ups, blood glucose monitoring, and pharmacotherapy.[55] This can lead to poor blood sugar control, an increased risk of diabetes-related complications, and poorer health outcomes.
Use of this content is subject to our disclaimer