Approach
The exact diagnosis of uveitis can be extremely challenging given the vast array of presentations and aetiologies. While clinical examination and laboratory testing are important for the diagnosis of uveitis, making a sound differential diagnosis of the underlying aetiology requires careful attention to the patient's presentation and history, and to demographic information.
Bilaterality is more commonly seen in systemic conditions.[2] Idiopathic, parasitic, herpetic, and post-surgical uveitis is more commonly unilateral.[23][24] Therefore, a bilateral versus unilateral presentation can assist in determining the aetiology.
The non-granulomatous versus granulomatous nature of the presentation will also help to narrow further the differential diagnosis of the underlying aetiology. Early referral to an ophthalmologist with experience in uveitis is essential.
Several pathological entities mimic uveitis (masquerade syndromes). Each may have an associated intra-ocular cellular reaction. None of these are related to either autoimmune inflammation or ocular infection. Broadly, they can be classified as neoplastic and non-neoplastic.
Ocular symptoms
Symptoms in uveitis generally depend on the location of the inflammation in the uvea, which is useful in differentiating between anterior and posterior uveitis. However, a thorough eye examination is the only way to confirm the diagnosis. A patient’s symptoms are less useful in further classifying the disease when dealing with possible intermediate or panuveitis.
Anterior uveitis
Patients with anterior uveitis often complain of ocular symptoms, including pain, redness, photophobia, and tearing. Patients may also have unilateral, dull pain that extends from the eye to the surrounding orbit. The area may be tender to palpation through the eyelid.
Common causes of anterior uveitis include human leukocyte antigen (HLA)-B27-associated iritis (including ankylosing spondylitis, psoriatic arthritis, and reactive arthritis), Behçet's disease, Fuchs' heterochromic iridocyclitis, and masquerade syndromes (disorders that may mimic uveitis).[11]
Posterior uveitis
In contrast, patients with posterior uveitis usually have a painless presentation. Decreased vision, floaters, photopsia (a perception of flashes of light), or asymptomatic presentation may occur in posterior uveitis, although this is highly variable depending on the location, severity, and aetiology of the inflammation.
Common causes of posterior uveitis include toxoplasmosis retinochoroiditis, herpes simplex virus (HSV) retinitis, tuberculosis (TB) choroiditis, sarcoidosis, and Vogt-Koyanagi-Harada syndrome (a multi-system autoimmune disorder characterised by chronic uveitis with skin, neurological, and auditory manifestations).[25]
Ocular symptom onset and duration
Onset and duration of the ocular symptoms offer clues to the aetiology.
Acute uveitis
Characterised by sudden onset (over hours or days) and limited duration (≤3 months' duration). Acute anterior uveitis is the most common form of acute uveitis. Causes of acute anterior uveitis include HLA-B27-associated disorders, systemic disease (e.g., sarcoidosis, Behçet's disease), infection, drug reactions, and trauma. Acute anterior uveitis may also be idiopathic, or mimicked by a masquerade syndrome.[17] Post-surgical bacterial endophthalmitis (a form of infectious panuveitis) also usually presents acutely.
Chronic uveitis
Defined as persistent uveitis (>3 months' duration) characterised by relapse within 3 months of therapy termination.[2] Several types of uveitis, such as tuberculous uveitis and Fuchs' heterochromic cyclitis, may present this way. Common causes of chronic anterior uveitis include systemic disease (e.g., sarcoidosis), Fuchs' heterochromic cyclitis, infection, chronic idiopathic anterior uveitis, and postoperative inflammation. Chronic anterior uveitis is not associated with HLA-B27.[17]
Chronic intermediate uveitis is often idiopathic, or may be associated with multiple sclerosis, sarcoidosis, syphilis, Lyme disease, or ocular lymphoma.[18]
Chronic posterior uveitis may be caused by an infection, including syphilis, TB, Lyme disease, or fungal, viral, or parasitic infection. It may be associated with many systemic diseases, including sarcoidosis, Behçet's disease, or Vogt-Koyanagi-Harada syndrome, or it may be associated with ocular syndromes such as sympathetic ophthalmia or birdshot chorioretinopathy.[18]
Recurrent uveitis
Characterised by repeated episodes separated by disease inactivity ≥3 months, whether on or off treatment; may be seen in many types of chronic uveitis, including Fuchs' heterochromic cyclitis and sarcoid-associated uveitis.
Systemic symptoms
Uveitis may occur as a manifestation of a disease with extra-ocular involvement, whether an infectious disease, an autoimmune-mediated systemic disease, or a masquerade syndrome.[11] Other uveitis syndromes may be confined primarily to the eye. Therefore, a thorough review of systems should help to narrow the possible aetiologies.
Symptoms such as pain or stiffness of the hands, wrists, fingers, toes, lower back, spine, or any weight-bearing joints should be sought. These symptoms may be found in patients with HLA-B27-associated disorders, rheumatoid arthritis, and systemic lupus erythematosus (SLE).
Rashes can be a systemic sign found in uveitis-associated diseases such as Lyme disease, syphilis, SLE, Kawasaki disease, and drug or hypersensitivity reactions.
Gastrointestinal disease, suggested by abdominal discomfort, nausea, vomiting, diarrhoea, or melaena, may point to Crohn's disease or ulcerative colitis. Behçet's disease and Whipple's disease may also have gastrointestinal manifestations.
Oral lesions (Behçet's disease, inflammatory bowel disease), genital lesions (Behçet's disease, syphilis), headache (Behçet's disease, Vogt-Koyanagi-Harada syndrome), neurological symptoms (multiple sclerosis, Behçet's disease), and pulmonary findings (TB) may also be useful in diagnosis.
Absence of systemic symptoms
May also prove useful in diagnosis. A lack of systemic findings is seen in uveitic syndromes confined primarily to the eye, including acute posterior multifocal placoid pigment epitheliopathy (APMPPE), Fuchs' heterochromic cyclitis, birdshot choroidopathy (a bilateral inflammatory disease affecting the choroid layer), and pars planitis.
[Figure caption and citation for the preceding image starts]: Comparison of common symptoms and causes of anterior and posterior uveitisCreated by John Huang, MD, CPE [Citation ends].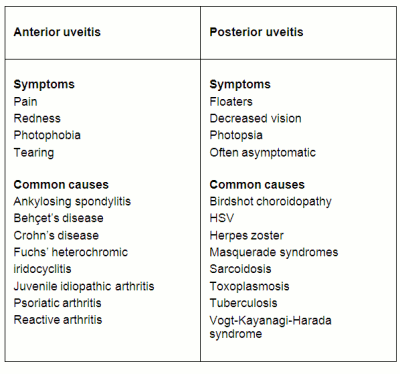
Comprehensive medical history
The patient’s medical history (including past ocular history, previous illnesses, and drugs) and family history may provide crucial information.
Any previous episode and treatment of uveitis should be documented to determine the duration of the disease and/or to determine if the current presentation is a relapse of an existing condition. Documentation of previous disease course and treatments may help to classify the disease and guide treatment. History of past ocular surgeries or trauma is also important. Existing ocular conditions including glaucoma, cataracts, and cystoid macular oedema should also be discussed and may be related to the current presentation.
Uveitis could be a manifestation of underlying disease. History of any autoimmune disease, infectious disease, or neoplastic and paraneoplastic disorders should be sought.
Medicines review
Careful review of a patient’s drug history may help with the diagnosis.
Drug-induced uveitis is relatively rare, but it may be seen in immunocompromised patients prescribed rifabutin as prophylaxis against atypical mycobacterial infections.[26] Cidofovir, an antiviral agent used to treat cytomegalovirus (CMV) in patients with AIDS, has also been shown to cause uveitis.[26] Protease inhibitors used in the treatment of AIDS have also been associated with uveitis.[27] Of note, HIV is a potential cause of anterior uveitis.[28] Therefore, determining the exact aetiology in patients with HIV who are prescribed any of these agents may be difficult.
Most drug-related uveitis respond well to topical corticosteroid treatment, often making the diagnosis of drug-induced uveitis possible shortly after initiation of treatment.
Family history
HLA-B27-associated iridocyclitis is seen in ankylosing spondylitis, reactive arthritis, and psoriatic arthritis. Inflammatory bowel disease is seen in families with HLA-B27 encoded in the major histocompatibility complex on chromosome 6.[29] Birdshot retinochoroidopathy is associated with HLA-A29.[30] Patients with known HLA correlations or those with a family history of an HLA-related disease may be at increased risk for associated conditions.
TORCH (Toxoplasmosis, Other agents, Rubella, CMV, HSV) syndrome covers the common list of perinatal infections associated with congenital infection and associated ocular manifestations. Toxoplasmosis is an example of a disease-causing uveitis by congenital transmission. This disease may be passed from mother to child, leading to ocular disease in the child. Additional common congenital infections include syphilis and HIV.
Demographic information in relation to disease risk
Age, sex, ancestry, social habits, sexual habits, and geographical and occupational exposure risks are important factors.
Patient age
May be the most useful demographic consideration when classifying uveitis, given that specific diseases often present in certain age groups.
Childhood uveitis, although less commonly seen than uveitis in older patients, may be seen in the paediatric population associated with juvenile idiopathic arthritis (JIA), HLA-B27-associated disorders, Lyme disease, toxocariasis, and retinoblastoma.[31]
In young adults, uveitis is most often associated with HLA-B27-associated disorders, Fuchs' heterochromic iridocyclitis, multiple sclerosis, Behçet’s disease, APMPPE, and pars planitis.
Middle-aged patients with uveitis often have ocular disease associated with sarcoidosis, SLE, birdshot retinochoroidopathy, and Vogt-Koyanagi-Harada syndrome.
Older adults rarely present with primary uveitis. This may be related to the overall decreased immune response in the older population. One of the rare instances of an autoimmune disease in older adults is giant cell arteritis. In patients with chronic persistent intra-ocular inflammation with poor response to treatment, intra-ocular lymphoma, other malignancies, and masquerade syndromes should be high on the list of possible causes.
Sex
Reactive arthritis, ankylosing spondylitis, and Behçet's disease are more commonly seen in men.
Men are more commonly found to have traumatic iritis, fungal endophthalmitis (a form of infectious panuveitis), and sympathetic ophthalmia, resulting from trauma or intravenous drug use.
SLE incidence is higher in women than in men; reported gender ratios range from 2:1 to 15:1.[32][33][34][35]
Ancestry
HLA-B27-associated iritis, including ankylosing spondylitis, reactive arthritis, and psoriatic arthritis, is more common in white people.
The lifetime risk of sarcoidosis for black people is slightly higher than for white people in the US.[36][37]
Behçet's disease is most commonly reported in the Middle East, the Mediterranean region, and eastern Asia. The prevalence is between 80-370 per 100,000 population in Turkey, 10 per 100,000 population in Japan, and 0.6 per 100,000 population in the UK.[38]
Vogt-Koyanagi-Harada syndrome is seen most commonly in Asian, Middle Eastern, Hispanic, and American Indian populations.[39]
Sexual and social habits
It is important to gather a full sexual history, including sexual practices, current genitourinary symptoms, and previous sexually transmitted infections. Consideration of sexual and social habits may uncover conditions that could have exposed the patient to infectious causes of uveitis.
Young, sexually active individuals and intravenous drug users are at increased risk for HIV infection and AIDS-related opportunistic infection. Common HIV-associated manifestations include CMV retinitis, Pneumocystis carinii choroiditis, TB-associated uveitis, toxoplasmosis retinochoroiditis, and candida endophthalmitis.
Sexually active people are more likely to acquire sexually transmitted infections, such as syphilis, gonorrhoea, and HSV, that can cause a primary infectious uveitis.
Chlamydia, the most common sexually transmitted infection, may trigger the HLA-B27-associated iritis in reactive arthritis.
Patients with a history of ingesting raw or undercooked pork are at increased risk of ocular toxoplasmosis. Patients who have ingested unpasteurised milk may be exposed to Brucella.
Patients with cats are at increased risk of exposure to faeces containing Toxoplasma gondii.
Ocular toxocariasis may be contracted from cats or dogs carrying this parasite.
Bartonella henselae, which causes cat-scratch disease, may also be transmitted to patients with exposure to cats.
Environmental exposure
Geographical location and history of recent travel may be important. Histoplasmosis is endemic to the Mississippi-Ohio-Missouri River valleys and must be considered in patients from that area and in those who have recently travelled there.
Coccidioidomycosis can be acquired by visiting or living in the south-western parts of the US.
Dysentery, TB, malaria, and leprosy may be acquired during travel or in endemic countries.
Patients who work on farms or work closely with animals are at higher risk for certain infectious diseases, including leptospirosis (seen in sewer or laboratory workers exposed to rat urine or contaminated water), brucellosis (seen in farm or slaughterhouse workers), and cat-scratch disease and toxoplasmosis (seen in patients who work with felines).
TB is commonly acquired from person-to-person transmission. It is easily spread through air droplets from a person with active disease.
Slit-lamp biomicroscopy and fundus examination
Slit-lamp biomicroscopy is used to identify the subclassification of uveitis: anterior, intermediate, posterior, or panuveitis.[3] Upon slit-lamp examination with tonometry, anterior uveitis may show conjunctival injection with exacerbation around the limbus (ciliary flush), a slight decrease in visual acuity, a decrease in intra-ocular pressure (rarely increased), lymphocyte aggregates in the corneal endothelium (keratic precipitates), corneal oedema, and white blood cells or protein in the anterior chamber (flare). Gonioscopy of both eyes should be performed on all patients in whom angle closure is suspected. Gonioscopy is used to visualise the anterior chamber angle of the eye, which is essential for evaluating and managing glaucoma.[40][41][42]
In order to determine the appropriate laboratory work-up in anterior uveitis, dilated fundoscopic examination is employed to differentiate granulomatous uveitis from non-granulomatous uveitis. The appearance of the keratic precipitates (KPs) aids in differentiation. Granulomatous uveitis has large 'mutton fat' KPs as well as clusters of cells in the anterior chamber (Busacca nodules), trabecular meshwork (Berlin nodules), or pupillary border (Koeppe nodules), whereas non-granulomatous uveitis has fine keratic precipitates over the corneal endothelium.
Intermediate and posterior uveitis require dilated fundoscopic examination to look for signs of vitritis (retinal exudates in the vitreous) and retinitis (retinal vascular sheathing, macular oedema, optic disc swelling, and retinal haemorrhages). In cases of chronic inflammation, a posterior subcapsular cataract may be visualised.
Fundus examination with fluorescein angiography is used to assess for retinal vascular leakage and/or non-perfusion (or other signs of inflammation) in retinal vasculitis; optical coherence tomography evaluates for macular oedema and glaucomatous progression.[3][41]
Establish location and type of uveitis
The initial focus is to narrow the work-up based on the location of the uveitis (anterior, intermediate, posterior, panuveitis) as well as the type of uveitis (non-granulomatous versus granulomatous). Possible causes classified by location and type include:
Anterior uveitis
Granulomatous
Infectious: viral (HSV and varicella zoster virus [VZV]), TB, syphilis, Lyme disease
Autoimmune: sarcoidosis, Vogt-Koyanagi-Harada syndrome, granulomatosis with polyangiitis, sympathetic ophthalmia
Non-granulomatous
HLA-B27-associated anterior uveitis, JIA-associated anterior uveitis, traumatic iritis, post-operative inflammation
With associated iris atrophy: viral (HSV, VZV, Epstein-Barr virus, CMV), syphilis, Fuchs' heterochromic iridocyclitis
With associated keratitis: viral (HSV, VZV), sarcoidosis, syphilis, SLE
With associated scleritis: viral (HSV, VZV), sarcoidosis, syphilis, SLE, granulomatosis with polyangiitis, polyarteritis nodosa, relapsing polychondritis
Intermediate uveitis
Infectious: Lyme disease, syphilis
Autoimmune: sarcoidosis, inflammatory bowel disease, multiple sclerosis, pars planitis
Posterior uveitis
Infectious: toxoplasmosis chorioretinitis, toxocariasis, acute retinal necrosis, CMV retinitis, syphilis
Autoimmune: serpiginous choroiditis, APMPPE, birdshot chorioretinopathy, multifocal choroiditis
Panuveitis
Infectious: syphilis, bacterial or fungal endophthalmitis (traumatic or endogenous)
Autoimmune: sarcoidosis, Vogt-Koyanagi-Harada syndrome, Behçet's disease, SLE, granulomatosis with polyangiitis
Laboratory evaluation is informed by potential causes of uveitis and presentation
Focus on finding infectious aetiologies, because they are often more aggressive and may lead to permanent blindness. Rule out infectious aetiologies before considering autoimmune-mediated aetiologies.
Syphilis infection
Rapid plasma reagin (RPR), venereal disease research laboratory (VDRL), and fluorescent treponemal antibody (FTA-ABS) testing can help to identify syphilis infection. Ocular manifestations of syphilis involve secondary, tertiary, and neurosyphilis; RPR and VDRL are usually positive in these patients. A positive FTA-ABS test result does not necessarily indicate active syphilis but a history of syphilis at some point, because the test remains positive for life after syphilis infection.
HSV, VZV, and CMV
Polymerase chain reaction may be considered to confirm suspected HSV, VZV, or CMV infection. Among viral causes, HSV and VZV are much more common.[15][16] Pain and blurring of vision may be more common in CMV anterior uveitis.[43]
First-episode unilateral, non-granulomatous uveitis without symptoms or systemic manifestations
No further evaluation is warranted. This is also true for patients with a recent history of trauma or surgery or with clinical signs of HSV or herpes zoster virus infection.
Bilateral, recurrent, or granulomatous uveitis
Full blood count, ACE level (may be elevated in sarcoidosis), syphilis serology, and HLA-B27 should be ordered to help identify a related systemic disease. Testing for rheumatoid factor and anti-cyclic citrullinated peptide antibodies should be done in the setting of possible rheumatoid arthritis. Antinuclear antibodies (ANA) and anti-double-stranded DNA (anti-dsDNA) antibodies are important diagnostic markers for SLE. Other HLA antigens may point to the presence of specific disorders. Elevated levels of anti-neutrophil cytoplasmic antibodies point to the presence of a vasculitic condition. Erythrocyte sedimentation rate, C-reactive protein, and urinalysis can be useful non-specific tests.
Investigations for autoimmune disease are conducted when infectious aetiologies have been excluded. The ophthalmologist may collaborate with a rheumatologist to investigate autoimmune-mediated aetiologies.
Consider predisposing risk factors
If the patient has a history of known exposure or is suspected to be at high risk as a result of occupation, lifestyle, or hobbies, relevant tests should be performed:
Lyme titre for Borrelia burgdorferi.
Purified protein derivative (PPD) skin testing for those with: HIV; a history of alcohol abuse; suspected TB or in an endemic region; recent contact with a person infected with TB. Screening tests to identify latent TB infection should be interpreted with caution, taking into account the person's immune status, history of exposure to TB and the Bacillus Calmette-Guérin (BCG) vaccination, and other risk factors. There is no reliable way to distinguish a positive TB skin test reaction caused by BCG vaccination from a reaction caused by true TB infection.[44]
Toxoplasma titre; ocular toxoplasmosis is one of the most frequent infectious aetiologies of posterior uveitis.[45]
Bartonella henselae titre, if cat-scratch disease is suspected.
Patients with retinal vasculitis: ANA and anti-neutrophil cytoplasmic antibodies are needed.
Diagnostic vitrectomy
Intra-ocular sampling, including diagnostic vitrectomy, may be of value in the setting of a diagnostic dilemma.[46] Diagnostic testing may include cytology, flow cytometry, Gram stain, culture (bacterial, fungal, and nocardial infection), polymerase chain reaction (PCR) testing (bacterial endophthalmitis and infection with mycobacterium tuberculosis; viral retinitides and ocular toxoplasmosis), antibody (toxoplasmosis and toxocariasis), and cytokine evaluation, depending upon the patient presentation.[46]
Use of this content is subject to our disclaimer