Approach
Hypogonadism is a common condition, but it is frequently unrecognised and undiagnosed because signs and symptoms may be non-specific, or overlap with other common conditions (such as depression, or ageing). It can occur at all stages of life from the postnatal period to old age.
Exact clinical features depend on the time of onset of testosterone deficiency, the testicular functions involved (testosterone production and/or spermatogenesis), and the site of dysfunction along the hypothalamic-pituitary-gonadal axis.
Diagnosis requires both the presence of clinical features and laboratory confirmation through appropriate laboratory testing, usually on two occasions (although a single sample will suffice if luteinising hormone [LH] is obviously raised).[1][2][7][Figure caption and citation for the preceding image starts]: An approach for the diagnostic evaluation of adult men suspected of having androgen deficiencyAdapted from Endocrine Society Guidelines, with permission [Citation ends].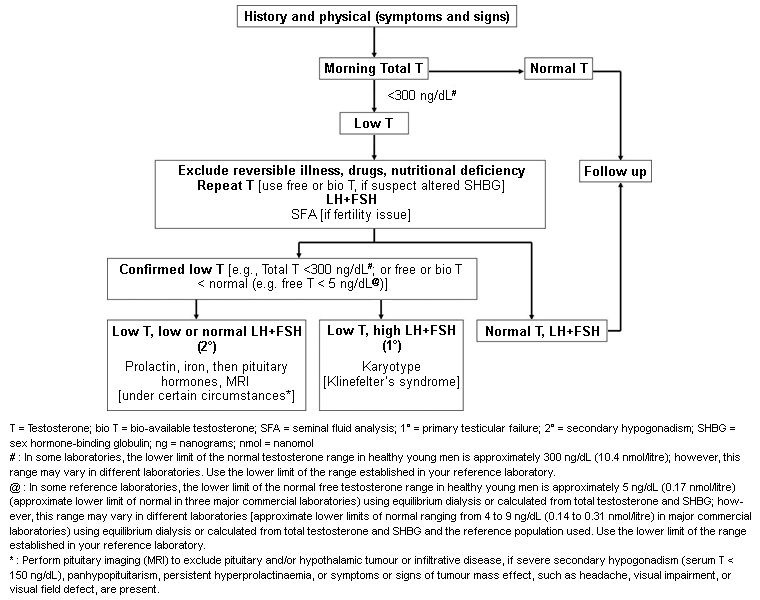
History
If hypogonadism started before puberty, adult patients typically report delayed or absent puberty/development of secondary male characteristics; infertility; and diminished libido.[7] They may give a history of sparse body and facial hair and a high-pitched voice compared with peers. Patients may report having a short penis and may have noted small testes, gynaecomastia, or give a history of cryptorchidism.[7] These features may be confirmed by examination, although great sensitivity should be shown and patients who already suffer from intense body shame may not wish to undergo a thorough genital examination.[8] Frequently, patients have segmental disproportion (long upper and lower limbs compared with truncal height) due to delayed pubertal closure of the epiphyses of the long bones.[7][34]
When hypogonadism occurs after a normal puberty, the presentation is frequently less formulaic. The main presenting symptoms are loss or reduction in libido, fatigue, erectile dysfunction, reduced physical strength/sarcopenia, gynaecomastia, osteoporosis, anaemia, infertility, or hot flushes/sweating.[7] Patients may report psychological symptoms, such as loss of motivation or concentration, irritability, low or labile mood, and body shame.[8] The external genitalia are usually normal in these patients, although testes may be soft and occasionally of reduced volume. There may be a history of testicular trauma or torsion, radiotherapy, treatment with alkylating agents, mumps, cryptorchidism, or some of the childhood developmental, behavioural, and/or educational issues characteristic of Klinefelter syndrome.[9][16]
The signs and symptoms that are particularly indicative of testosterone deficiency include:[7]
Incomplete sexual development, eunuchoidal proportions
Loss of libido
Decrease in spontaneous early morning erections
Erectile dysfunction
Subfertility, low or zero sperm count
Decreased muscle mass and strength
Mild, normochromic, normocytic anaemia
Loss of height, low trauma fracture, low bone mineral density/osteoporosis
Hot flushes, sweats
Breast discomfort and gynaecomastia
Loss of axillary and pubic hair; reduced shaving
Very small or shrinking testes (especially <5 mL each).
Other more general signs and symptoms that may also be associated with testosterone deficiency include:
Decreased energy, motivation, or initiative
Diminished physical or work performance
Low mood or emotional lability
Poor concentration and memory
Sleep disturbance
Increased body fat, increased BMI, central obesity.
Examination
When a diagnosis of hypogonadism is suspected, a thorough and careful examination should be undertaken, subject to patient sensitivities, focused on the features discussed above.
General examination should focus on the body habitus of the patient. A low BMI suggests the possibility of relative energy deficit due to imbalance between exercise and food intake; potentially behavioural, but also raising the possibility of coeliac disease or other cause of malabsorption.[7] Tall men should be carefully assessed for segmental disproportion (i.e., arm span >5 cm greater than height, or sitting height <50% of standing height).[7] These proportions develop due to a delay in the fusion of the epiphyses of the long bones, caused by a deficiency of oestradiol arising as a result of testosterone deficiency.[34] Absence of facial hair and a lower hairline may also be noted in many men with hypogonadism. Some men may have a high-pitched voice.
Evaluation of visual fields and extraocular movements may be important because a pituitary tumour may compress the optic chiasm. Assessing visual fields by red target confrontation is a sensitive test for bitemporal hemianopia, but confrontation by finger-wagging much less so. Formal testing of perimetry in an ophthalmic setting is preferred.
Anosmia, cleft lip or palate, hearing impairment, synkinesia, renal aplasia, or syndactyly/clinodactyly may point towards congenital hypogonadotrophic hypogonadism.[2]
Gynaecomastia may be present. This is firm, ductal tissue that is mainly subareolar. It is more commonly seen in primary hypogonadism.[35] It should be differentiated from adipose tissue in obese patients (often termed lipomastia or pseudogynaecomastia).
Fine diagonal wrinkling of the facial skin occurs with prolonged and severe hypogonadism.
Permission should be sought for external genital examination to evaluate for micropenis, scrotal hypoplasia, or cryptorchidism. Normal testes measure 12 to 25 mL on the Prader orchidometer, but most clinicians tend to overestimate compared with dimensions obtained with ultrasound.[36] If the patient prefers, they may be given an orchidometer and clear instructions to estimate their own testicular volume. Presence of the vas deferens can be confirmed by palpation of the spermatic cord.
Measurement of serum testosterone levels
Laboratory investigation of hypogonadism focuses on evaluating serum testosterone levels and performing seminal fluid analysis, guided by presenting symptoms and whether fertility and/or androgenisation is the treatment goal.
The majority of circulating testosterone is bound to protein. Approximately 65% is strongly bound to sex hormone-binding globulin (SHBG) and is not bioactive. A further approximate 33% of testosterone is weakly bound to albumin; this portion can rapidly dissociate from the albumin and so is bioactive.[4] Approximately 1% to 4% is unbound (free testosterone) and is also bioactive.[4] The albumin-bound and free testosterone are collectively known as bioavailable testosterone. The half-life of free testosterone is 10 minutes.
A number of tests are available for measuring these various components.
Total serum testosterone (free and bound): can be determined by enzyme-linked immunosorbent assay (ELISA) or mass spectroscopy. This test is readily available, reliable, and is comparatively inexpensive. It is generally considered the first-line investigation in the evaluation of suspected hypogonadism.
Bioavailable testosterone (i.e., non-SHBG-bound and free testosterone): can be measured using a 50% ammonium sulphate precipitation technique. This test is cumbersome and unsuited to routine use.
Free testosterone: can be measured by equilibrium dialysis, but this is not widely available. Free testosterone immunoassay is inaccurate.
Calculated free and bioavailable testosterone levels: can be performed using validated equations based on the total testosterone, SHBG, and, in some equations, the albumin level. The Vermeulen equation for free testosterone is the most commonly used.
Total serum testosterone is the usual first-line test (although evaluating free or bioavailable testosterone levels may be necessary in some patients). Fasting morning total testosterone should be measured on at least two occasions, 1 week apart (unless gonadotrophins are raised or there is an obvious underlying cause of hypogonadism, e.g., pituitary macroadenoma).[1][37] The timing of sampling is important, especially in younger men. Testosterone levels follow a circadian rhythm, reaching a maximum at 8 a.m. and declining to a minimum at 10 p.m. (this pattern is less marked in older men, although some older men do maintain a normal rhythm). Because reference ranges are based on peak levels in normal young men, it is best if samples are taken between 6 a.m. and 8 a.m. However, for practical reasons, a sample taken up to 11 a.m. is acceptable. Ensure that samples are taken fasted to eliminate the acute effect of protein-calorie intake to inhibit gonadotrophin-mediated testosterone secretion.[2]
Confirm low testosterone concentrations in men with an initial testosterone level in the mildly hypogonadal range, because 30% of such patients may have a normal testosterone level on repeat measurement.[38] Taking testosterone samples during periods of acute illness, shift work, or sleep deprivation should be avoided as these can transiently suppress the hypothalamic-pituitary-testes axis and cause inaccuracies in evaluation.
Serum sex hormone-binding globulin (SHBG) should be checked in men with an equivocal or borderline total testosterone. Measurement of SHBG allows calculation of free testosterone.[37][39][40] In men with elevated or with low SHBG levels (in whom total testosterone will necessarily underestimate or overestimate androgenicity, respectively), calculating free testosterone level is diagnostically useful.[37][40] For example:
Older men often have increasing levels of SHBG with increasing age, which causes a low free testosterone with a normal-appearing total testosterone.
Obese men often have low SHBG levels, and may have normal free testosterone even when total appears low.
Note that inter-assay variation in SHBG levels is much higher than for testosterone.[2][29][37][39][40]
Total testosterone thresholds
Many experts and guidelines suggest a total testosterone level <10.4 nmol/L (<300 ng/dL) as the threshold for hypogonadism (using a US standardised assay).[1] Below this level there is evidence of bone loss and increased fat accumulation. Assay standardisation between laboratories is encouraged.[41][42]
Some European guidelines consider a total testosterone level <8 nmol/L (<230 ng/dL) to be indicative of hypogonadism, with level >12 nmol/L (>350 ng/dL) not usually consistent with hypogonadism.[2][29][40] Intermediate results require careful clinical consideration.
Measurement of gonadotrophins
If testosterone levels are found to be consistently low, LH and follicle-stimulating hormone levels should be measured to determine whether the patient has primary or secondary hypogonadism.[37] Gonadotrophin levels should be checked when clinical indicators of hypogonadism are strong, or many cases of Klinefelter syndrome will be missed.
Elevated gonadotrophin levels combined with low testosterone levels confirms primary hypogonadism. In these patients, karyotyping should be considered to evaluate for Klinefelter syndrome.[7]
Low or inappropriately normal gonadotrophin levels combined with low testosterone levels suggests either secondary hypogonadism, non-gonadal illness, or non-optimal (e.g., post-prandial) venepuncture conditions. Further testing for cause is required for these patients.
Semen analysis
Semen analysis is performed in men who have low testosterone levels and report infertility, or who express a desire to start a family in the future. A normal semen analysis makes the diagnosis of hypogonadism very unlikely.
Two semen analyses (each with samples obtained after 2-7 days of abstinence) are recommended to make an accurate diagnosis. If a semen volume of less than 1 mL is seen on analysis, ask the patient if the entire sample was collected in the cup.
Normal semen quality is sperm concentration >16 million/mL, total sperm motility >40%, and normal morphology >4% by Kruger strict criteria. Based on the World Health Organization reference ranges, a sperm count <16 million/mL is considered oligospermic; a count of <5 million/mL suggests severe oligozoospermia.[43] Men with azoospermia should be referred for urological assessment. They should undergo karyotyping to evaluate for Klinefelter syndrome, Y-chromosome microdeletion analysis, and cystic fibrosis transmembrane receptor (CFTR) mutations, which may cause congenital bilateral absence of the vasa deferentia.
Further investigations
A full blood count is recommended. Normochromic normocytic anaemia is a typical feature in all forms of male hypogonadism.[7] In general, a low or low-normal haemoglobin or haematocrit supports the diagnosis of hypogonadism, whereas high or high-normal level does not.
Karyotype or copy number variation test for Klinefelter syndrome
In men with low testosterone and elevated gonadotrophin levels (primary hypogonadism) and very small testes, a karyotype or copy number variation test should be ordered to diagnose Klinefelter syndrome.[7] See Klinefelter syndrome.
Testing for causes of secondary hypogonadism
If results of testosterone and gonadotrophin testing suggest secondary hypogonadism (i.e., low testosterone, accompanied by low or inappropriately normal gonadotrophins), then further testing for cause is required. This includes serum prolactin (to exclude hyperprolactinaemia) and iron saturation (to screen for iron overload syndromes, such as haemochromatosis).[7] MRI pituitary may be considered to exclude pituitary and/or hypothalamic tumours or infiltrative disease. The optimal strategy for pituitary imaging is uncertain.[44] Assessment of other pituitary hormones may be required if suggested by the clinical scenario.
Bone mineral density
Long-standing hypogonadism is associated with osteoporosis that can result in fractures occurring with unusually low levels of trauma. Men who have had, or are at risk for, falls and bone fractures should have an assessment of their bone mineral density.
Use of this content is subject to our disclaimer