Criteria
Biopsy determination of grade and stage
Pathology reports on biopsy and surgical specimens should include histological subtype, grade, and depth of invasion. Treatment is guided primarily by tumour stage and grade.
Stage
The tumour-nodes-metastases staging system is most widely used.[3][4] Non-muscle-invasive bladder cancer (NMIBC) includes carcinoma in situ (CIS; Tis) and papillary or solid tumours confined to the urothelium (Ta) and invading the lamina propria (T1). Approximately 75% to 80% of bladder tumours present as non-muscle-invasive disease and the remainder present as muscle-invasive disease.[2] In non-muscle-invasive disease, approximately 70% present as Ta lesions, 20% as T1 lesions, and 10% present as CIS (Tis) lesions.[2]
T2 tumours invade muscularis propria. Tumours extending into perivesical fat are T3 and those extending into adjacent organs are T4. Nodal metastasis (N) and extrapelvic metastasis (M) complete the system.
Grade
The 1973 World Health Organization graded urothelial carcinoma as well differentiated (grade 1), moderately differentiated (grade 2), and poorly differentiated (grade 3).[6] In 2004, WHO along with the International Society of Urological Pathology amended the grading system to create 3 grades: i) papillary urothelial neoplasia of low malignant potential, ii) low-grade urothelial carcinoma, and iii) high-grade urothelial carcinoma.[5][7]
American Urology Association guidelines use the WHO/International Society of Urological Pathology 2004 grading system.[60]
European Association of Urology guidelines suggest using both the 1973 and 2004/2022 grade systems concurrently, or a hybrid system.[21] High-grade tumours (including all grade 3 and CIS and some grade 2 WHO 1973) are aggressive tumours that if not controlled by intravesical therapy are a threat to life.
American Urological Association/Society of Urologic Oncology (AUA/SUO) risk stratification for NMIBC
AUA/SUO guidelines use the following definitions for risk in NMIBC:[60]
Low risk: solitary, small-volume (≤3 cm), low-grade Ta disease (Ta = non-invasive papillary carcinoma); any papillary urothelial neoplasm of low malignant potential.
Intermediate risk: solitary, large-volume (>3 cm), low-grade Ta disease; multi-focal low-grade Ta disease; high-grade Ta disease ≤3 cm; low-grade T1 disease (T1 = tumour invades subepithelial connective tissue, i.e., the lamina propria); or recurrence of low grade Ta tumour within 1 year.
High risk: carcinoma in situ (CIS; Tis); high-grade Ta >3 cm or multifocal; high-grade T1; any recurrent high-grade Ta tumour; any BCG failure in a high-grade patient; any subtype (variant) histology or lymphovascular or prostatic urethral invasion.
The AUA/SUO system is intended to guide treatment and surveillance decisions based on prognosis. It includes consideration of prior BCG intravesical therapy.[60] One retrospective review of patients treated between 2001 and 2017 found that the AUA/SUO system appropriately stratified patients for likelihood of recurrence and progression.[89]
NCCN guidelines recommend management of NMIBC based on the AUA/SUO risk stratification system, but stress that individual patients may have more or less concerning features that may affect management decisions.[61]
European Organisation for Research and Treatment of Cancer (EORTC) scoring system
Heterogeneity in bladder tumours complicates the ability to compare the efficacy of different treatment modalities and thereby establish unified treatment recommendations. Therefore, risk stratification is imperative for classifying patients with similar risks of recurrence and progression and helps to determine the appropriate management strategies for each risk category.
The EORTC risk tables are considered useful for estimating progression and/or recurrence of NMIBC. The scoring system combines data on previous tumour recurrence rate, number of tumours, tumour diameter, T category, and WHO grade, and the presence or absence of concomitant carcinoma in situ, to estimate risk of recurrence and progression.[90]
The EORTC risk tables were, however, developed using trial data from patients treated with older intravesical chemotherapy regimens (that do not reflect current standards of care). Bacillus Calmette-Guérin (BCG) therapy was infrequently used in the trials that informed the EORTC risk tables. With the subsequent increased use of BCG, the tables overestimate risk of recurrence and progression after BCG therapy.[91]
[Figure caption and citation for the preceding image starts]: European Organisation for Research and Treatment of Cancer (EORTC) scoring system: weighting used to calculate recurrence and progression scores. CIS, carcinoma in situFrom World J Urol. 2007 Jun;25(3):285-95; used with permission [Citation ends].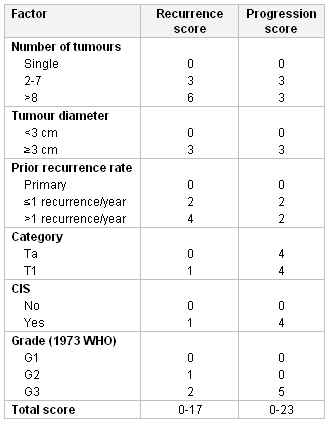
[Figure caption and citation for the preceding image starts]: European Organisation for Research and Treatment of Cancer (EORTC) risk tables: probability of recurrence and progression according to scoreFrom World J Urol. 2007 Jun;25(3):285-95; used with permission [Citation ends].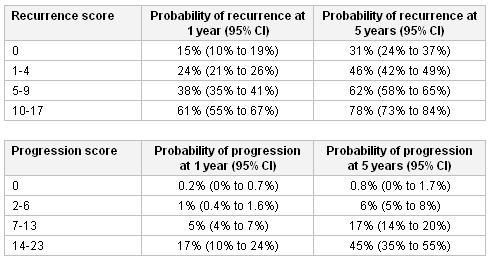 The Web-based EORTC risk calculator based on these tables can be downloaded and used to determine the most appropriate treatment strategies based on risk of progression and/or recurrence.
European Organisation for Research and Treatment of Cancer (EORTC) scoring system and risk tables for stage Ta T1 bladder cancer
Opens in new window
The Web-based EORTC risk calculator based on these tables can be downloaded and used to determine the most appropriate treatment strategies based on risk of progression and/or recurrence.
European Organisation for Research and Treatment of Cancer (EORTC) scoring system and risk tables for stage Ta T1 bladder cancer
Opens in new window
Use of this content is subject to our disclaimer