Summary
Definition
History and exam
Key diagnostic factors
- presence of risk factors
- history of recurrent or severe bleeding
- bleeding into muscles
- prolonged bleeding following heel prick or circumcision
- mucocutaneous bleeding
- haemarthrosis
- pseudotumour
- intracranial bleeding
Other diagnostic factors
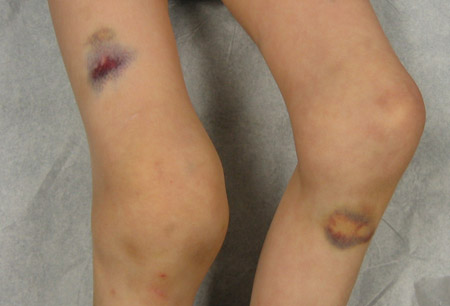
- excessive bruising/haematoma
- fatigue
- menorrhagia and bleeding following surgical procedures or childbirth (female carriers)
- extensive cutaneous purpura (acquired haemophilia)
- gastrointestinal bleeding and haematuria
- distended and painful abdomen
- pallor, tachycardia, tachypnoea, or hypotension
Risk factors
- family history of haemophilia (congenital haemophilia)
- male sex (congenital haemophilia)
- age >60 years (acquired haemophilia)
- autoimmune disorders, inflammatory bowel disease, diabetes, hepatitis, pregnancy and postnatal period, malignancy, monoclonal gammopathies, use of certain drugs (acquired haemophilia)
Diagnostic investigations
1st investigations to order
- activated partial thromboplastin time (aPTT)
- plasma factor VIII and IX assay
- mixing study
- FBC
- prothrombin time (PT)
- plasma von Willebrand factor assay
- plasma factor V, VII assay
- plasma factor XI, XII assay
- closure time/bleeding time and platelet aggregation studies
- serum liver aminotransferases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT])
- plain x-rays of specific bony sites
- antenatal factor VIII or IX mutation analysis by amniocentesis or chorionic villus sampling (CVS)
Investigations to consider
- head or neck CT
- head or neck MRI
- abdominal ultrasound or abdominopelvic CT scan
- oesophagogastroduodenoscopy or colonoscopy
- blood factor VIII or IX mutation analysis
- plasma factor VIII or IX inhibitor screen
- Bethesda assay/modified Bethesda assay (on plasma sample)
Treatment algorithm
Contributors
Authors
Man-Chiu Poon, MD, FRCP (C), FACP
Professor Emeritus
Departments of Medicine, Pediatrics and Oncology
Cumming School of Medicine
University of Calgary
Calgary
Canada
Disclosures
M-CP has been an ad hoc speaker for Bayer, Novo Nordisk, and Pfizer; attended advisory board meetings of Bioverativ/Sanofi, CSL Behring, KVR Pharmaceuticals, Novo Nordisk, Octapharma, Pfizer, Roche, and Takeda; received grant funding from Bayer and CSL Behring; and undertaken contract research for Novo Nordisk.
Adrienne Lee, MD, FRCP (C)
Clinical Assistant Professor
Department of Medicine
Cumming School of Medicine
University of Calgary
Calgary
Canada
Disclosures
AL declares that she has no competing interests.
Acknowledgements
Professor Poon and Dr Lee would like to gratefully acknowledge Dr Nigel S. Key, Dr Paul Giangrande, Dr Nidra I. Rodriguez, and Dr W. Keith Hoots, the previous contributors to this topic.
Disclosures
NSK has undertaken paid consultancy for Baxter Biosciences, Novo Nordisk, CSL Behring, and Bayer. He has received grant funding from Baxter. PG has undertaken paid consultancy and/or received lecture fees from the following companies involved in haemophilia care: Bayer, CSL Behring, NovoNordisk, Pfizer/ BPL, Octapharma, Biogen Idec, and Biotest. NSK, NIR, and WKH are authors of reference(s) cited in this topic.
Peer reviewers
Louis Aledort, MD
The Mary Weinfeld Professor of Clinical Research in Hemophilia
Mount Sinai School of Medicine
New York
NY
Disclosures
LA declares that he has no competing interests.
Christoph Pechlaner, MD
Associate Professor of Medicine
Innsbruck Medical University
Innsbruck
Austria
Disclosures
CP declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020 Aug;26 Suppl 6:1-158.Full text Abstract
Collins PW, Chalmers E, Hart DP, et al. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: 4th edition. Br J Haematol. 2013 Jan;160(2):153-70.Full text Abstract
National Hemophilia Foundation Medical and Scientific Advisory Council (MASAC). MASAC Document 280 - MASAC recommendations concerning products licensed for the treatment of hemophilia and selected disorders of the coagulation system. Aug 2023 [internet publication].Full text
National Hemophilia Foundation Medical and Scientific Advisory Council (MASAC). Guidelines for emergency department management of individuals with hemophilia and other bleeding disorders. Dec 2019 [internet publication].Full text
Tiede A, Collins P, Knoebl P, et al. International recommendations on the diagnosis and treatment of acquired hemophilia A. Haematologica. 2020 Jul;105(7):1791-801.Full text Abstract
Reference articles
A full list of sources referenced in this topic is available here.
Use of this content is subject to our disclaimer