Recommendations
Key Recommendations
Suspect aortic dissection if the patient presents with chest, back, or abdominal pain that has an abrupt onset, is severe in intensity, or is described as sharp' or 'stabbing' and maximal at onset, particularly if they have any high-risk conditions such as:[2][15]
Marfan syndrome (or other connective tissue diseases)[17]
A family history of aortic disease
Known aortic valve disease or thoracic aortic aneurysm
Previous aortic manipulation (including cardiac surgery)[17]
Always take a comprehensive acute history;missing the diagnosis of aortic dissection may be catastrophic.[15] Although a typical patient with aortic dissection is a male in their 50s, always consider the diagnosis in younger patients even if they have no risk factors.
Always organise ECG, chest x-ray (if the patient’s clinical status allows), and blood tests (to detect complications of aortic dissection or other differentials) initially.[2][6][17] The European Society of Cardiology (ESC) recommends transthoracic echocardiography (TTE) should also be used as an initial investigation.[2][29] However, TTE is not widely available in many emergency departments in the UK.[2][17] Further imaging for definitive diagnosis is also required urgently; computed tomography (CT) is used as first line (and as an alternative to TTE).[14] Trans-oesophageal echocardiography (TOE) and magnetic resonance imaging (MRI) are alternatives.[2][14][17]
Choice of imaging will depend on the patient’s haemodynamic status, the likelihood of aortic dissection, and local availability and expertise.[2][Figure caption and citation for the preceding image starts]: Multiparametric diagnostic work-up of acute aortic syndromeMazzolai L et. al, 2024 ESC guidelines for the management of peripheral arterial and aortic diseases: developed by the task force on the management of peripheral arterial and aortic diseases of the European Society of Cardiology (ESC) endorsed by the European Association for Cardio-Thoracic Surgery (EACTS), the European Reference Network on Rare Multisystemic Vascular Diseases (VASCERN), and the European Society of Vascular Medicine (ESVM), 45(36), 3538-700, https://doi.org/10.1093/eurheartj/ehae179. Reprinted by permission of Oxford University Press on behalf of the European Society of Cardiology. http://www.escardio.org/Guidelines [Citation ends].
Suspect aortic dissection if the patient presents with chest, back, or abdominal pain that has an abrupt onset, is severe in intensity, or is described as sharp' or 'stabbing' and maximal at onset, particularly if they have any high-risk conditions such as:[2][15]
Marfan syndrome (or other connective tissue diseases)[17]
A family history of aortic disease
Known aortic valve disease or thoracic aortic aneurysm
Previous aortic manipulation (including cardiac surgery)[17]
Other risk factors include:
Hypertension[17]
This is the most important predisposing factor for aortic dissection; it has been reported in 77% of patients with aortic dissection and is often poorly controlled.[16]
Male sex[17]
Older age[17]
Recent history of heavy lifting[17]
Use of cocaine[17]
Always take a comprehensive acute history.
Practical tip
Missing the diagnosis of aortic dissection may be catastrophic. Although a typical patient with aortic dissection is a male patient in their 50s, always consider the diagnosis in younger patients even if they have no risk factors.
Chest pain is the most common symptom.[2][17]
Although the classic textbook description is of acute 'tearing' or 'ripping' pain, patients more commonly report the abrupt onset of severe 'sharp' or 'stabbing' pain, maximal at onset.[6]
The pain associated with aortic dissection may be located retrosternally, interscapularly, or in the lower back.
Anterior chest pain is typically associated with an ascending dissection; interscapular pain usually occurs with a descending dissection.
Pain may migrate through the thorax or abdomen, and the location of pain may change with time as the dissection extends.[17]
A minority of patients present with syncope, or without pain. It is important to recognise that no single symptom of aortic dissection is pathognomonic for the condition, as there is overlap with cardiac, pulmonary, abdominal, and musculoskeletal disorders.[15]
Symptoms of visceral or extremity ischaemia may also be present.
If the patient presents with an incidental finding of chronic dissection on imaging, ask about:[2]
History of a previous acute pain event. However, acute chest pain may also indicate rupture.
Symptoms related to an enlarging dissection (such as hoarseness or new onset of chest pain)
Assess the patient urgently for signs of shock, heart failure, and cardiac tamponade. See Shock, Acute heart failure, and Cardiac tamponade.
However, be aware that some patients with dissection may be haemodynamically stable.[6]
Check for evidence of a perfusion deficit, which includes:
A pulse deficit (reduction or absence of a pulse)[30]
A pulse difference in the lower limbs may also be evident, and in some cases may lead to acute limb ischaemia.
Systolic blood pressure difference between the patient’s arms[2]
Measure blood pressure in both arms. A difference in systolic blood pressure of greater than 20 mmHg between the two arms is a key sign of aortic dissection.[6]
A focal neurological deficit (in conjunction with pain), which includes:[2]
Paraesthesia
Weakness
Paraplegia
Auscultate the chest; a diastolic decrescendo murmur may be heard which indicates aortic insufficiency.
Other features of aortic dissection include:
Reduced conscious level
A left pleural effusion - see Pleural effusion.
If a patient presents with an incidental finding of chronic dissection, check for chronic malperfusion (abdominal pain, claudication).[2]
Choice of investigations for diagnostic work-up of acute dissection is based on the patient’s haemodynamic status, likelihood of aortic dissection, and local availability and expertise.[2]
In patients presenting with chest pain, a routine chest radiography and ECG are recommended to exclude other aetiologies; however, the absence of these findings should not delay further investigations.[2][6][17][31] Laboratory tests should be obtained, but awaiting results should not delay imaging if there is a high probability of aortic dissection.[2]
Although the ESC recommends that transthoracic echocardiography (TTE) should be used during initial investigation. TTE is not widely available in emergency departments in the UK. In practice, computed tomography (CT) may be used initially if the patient is haemodynamically stable and TTE is unavailable or inconclusive.[2][17]
If the patient is haemodynamically unstable, seek help from a senior colleague. Confirm the diagnosis using CT.[14] Trans-oesophageal echocardiography (TOE) is an alternative to CT in unstable patients who cannot be transferred to CT.[2][14]
If the patient is haemodynamically stable and you have a high suspicion of dissection, organise urgent CT for definitive diagnosis.[14] TOE and MRI are alternatives.[2][14][17]
If the patient presents with an incidental finding of chronic dissection (such as mediastinal widening or prominent aortic knob on chest x-ray), diagnosis is confirmed by cross-sectional imaging such as contrast-enhanced CT, TOE, or MRI.[2]
ECG
Always perform an ECG in any patient with acute chest pain to rule out ST-elevation myocardial infarction.[2][17][31] See ST-elevation myocardial infarction.
However, be aware that myocardial ischaemia or infarction may be present in patients with aortic dissection, which can mask the diagnosis of dissection.[2] This may be due to:
Aortic false lumen expansion causing compression or obliteration of the coronary ostia
Propagation of the dissection into the coronary arteries
Echocardiography
Organise TTE for all patients with suspected acute aortic dissection, depending on local availability and expertise.[2][17] If the patient is haemodynamically unstable, get help from a senior colleague.[2][33][31]
Practical tip
In the UK, TTE is not available in many emergency departments and may give poor quality images.[17] In practice, organise CT if TTE is unavailable or inconclusive and the patient is haemodynamically stable.
Consider TOE for definitive diagnosis of acute aortic dissection in addition to TTE, depending on local expertise and availability.[2] In unstable patients who cannot be transferred to CT, TOE is recommended for diagnosis.[2]
For type A dissections (ascending), TOE may be performed in the ICU or operating theatre to confirm the diagnosis and better evaluate the aortic valve.
Sensitivity and specificity for TOE are higher than for TTE.
CT and MRI are alternatives, depending on local availability and the patient’s haemodynamic status.[2]
TOE can also be used to confirm the diagnosis of chronic dissection.[2][Figure caption and citation for the preceding image starts]: Trans-oesophageal echocardiography (transverse aortic section) showing a circumferential dissection of the ascending aorta in a 30-year-old patient with features of Marfan's syndromeBouzas-Mosquera A, Solla-Buceta M, Fojón-Polanco S. Circumferential aortic dissection. BMJ Case Reports 2009; doi:10.1136/bcr.2007.049908 [Citation ends].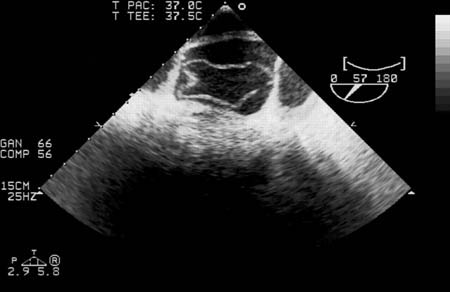
Chest x-ray
Order a chest x-ray for all patients, if the clinical situation allows.[6]
If chest x-ray shows widening of the mediastinum or pleural effusion, this can indicate aortic dissection.[14] Organise further definitive imaging with CT. [14] TOE and MRI are alternatives.[2][34] However, if you have a high suspicion of aortic dissection, do not delay definitive imaging for aortic dissection, even if the chest x-ray is normal.[6]
Chest x-ray is of limited value for diagnosis of aortic dissection, particularly if the dissection is confined to the ascending aorta; it is normal in up to 40% of patients with aortic dissection.[17][35]
Chest x-ray may also show other causes of acute chest pain.[2]
Computed tomography (CT)
Use urgent CT from neck to pelvis as first-line imaging for definitive diagnosis of aortic dissection.[2][14][31] TOE and MRI are alternatives, depending on local expertise and availability and the patient’s haemodynamic status.[2][17]
In practice, CT may also be used as initial imaging for suspected aortic dissection instead of TTE if this is unavailable or inconclusive, and the patient is haemodynamically stable.
A protocol of non-enhanced CT, followed by contrast-enhanced CT angiography is recommended.[2] This should include CT of the chest, abdomen, and pelvis to visualise the extent of the dissection.
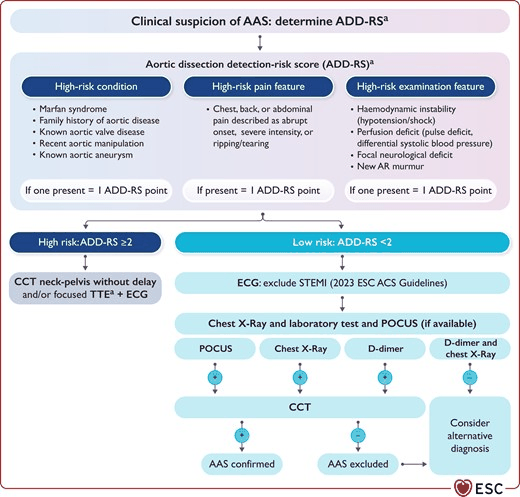
The ESC recommends that contrast CT neck-pelvis should be performed without delay and/or focused TTE and ECG if acute aortic syndrome is suspected and a patient is calculated to have an aortic dissection detection-risk score (ADD-RS) of ≥2 (high risk) using the following criteria:
High-risk condition: if one present = 1 ADD-RS point
Marfan syndrome; family history of aortic disease; known aortic valve disease; recent aortic manipulation; known aortic aneurysm
High-risk pain feature: if one present = 1 ADD-RS point
Chest, back, or abdominal pain described as abrupt onset, severe intensity, or ripping/tearing
High-risk examination feature: if one present = 1 ADD-RS point
Haemodynamic instability (hypotension/shock); perfusion deficit (pulse deficit, differential systolic blood pressure); focal neurological deficit; new aortic regurgitation murmur
[Figure caption and citation for the preceding image starts]: CT of a 71-year-old man showing type II dissecting aneurysm of the ascending aorta. Haematoma around the proximal segment of the ascending aorta (panels A-D) compressed the right pulmonary artery, almost occluding its patency and limiting the perfusion of the reciprocal lung [Citation ends].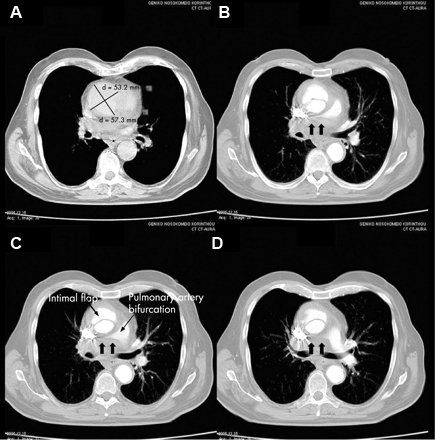 [Figure caption and citation for the preceding image starts]: CT scan showing dissecting aneurysm in a 45-year-old patient with Marfan syndrome experiencing chest pain [Citation ends].
[Figure caption and citation for the preceding image starts]: CT scan showing dissecting aneurysm in a 45-year-old patient with Marfan syndrome experiencing chest pain [Citation ends].
Blood tests
Order a D-dimer (preferably point-of-care) if you have a low clinical suspicion of aortic dissection.[2]
Despite a high sensitivity, D-dimer is not recommended as the sole screening tool for acute aortic dissection; while negative D-dimer may be helpful to rule out aortic dissection in a patient with a low clinical suspicion of dissection, a positive D-dimer lacks specificity when used in isolation.[36][37]
However, D-dimer is useful when considering the differential diagnosis (e.g., pulmonary embolism).[17][38]
When D-dimer levels are below 500 ng/mL, acute aortic dissection is unlikely.[2][39]
Request a group and save +/- crossmatch if surgical intervention/transfusion is necessary.[2]
Order the following blood tests in all patients to detect complications of aortic dissection (such as malperfusion) or other differentials:[2][40]
Full blood count
C-reactive protein
High sensitivity troponin
Urea, creatinine, and electrolytes
Liver function tests
Lactate
Blood gas
Creatine kinase
Procalcitonin
Coagulation studies
More info: Biomarkers
Several biomarkers show potential to assist in the diagnosis of aortic dissection, including smooth muscle myosin, calponin, matrix metalloproteinase 8, and soluble elastin fragments. These relate to injury of:
The vascular endothelial or smooth muscle cells (smooth muscle myosin)
The vascular interstitium (calponin, matrix metalloproteinase 8)
The elastic laminae (soluble elastin fragments).
However, none of these have currently been validated and they are not in use in clinical practice.[41]
Magnetic resonance angiogram
Magnetic resonance angiogram is the most accurate, sensitive, and specific test for aortic dissection, but is rarely used in the acute setting because it is more difficult to obtain than CT.[2][14][17] It should only be used if the patient is haemodynamically stable; CT and TOE are alternatives depending on local expertise and availability.[2][14][17]
Intravascular ultrasound
If the patient has a dissection and surgery is required, intra-operative intravascular ultrasound may be a useful adjunct in defining the morphology of the dissection and assists in the treatment plan, particularly during endovascular treatment.[14][17]
Use of this content is subject to our disclaimer