Approach
The diagnosis of acute myeloid leukaemia (AML) requires a multi-faceted approach that includes medical history, physical examination, laboratory tests, bone marrow evaluation (including cytomorphology assessment and immunophenotyping), and genetic testing.[27][45]
As AML and acute lymphoblastic leukaemia (ALL) are often clinically indistinguishable, confirmation of a myeloid origin of the leukaemic cells by immunophenotyping is essential. This may be done on peripheral blood before confirmation from the bone marrow.
History
In all patients, a complete medical history (including family history, if known) is important for diagnosis.
Risk of AML is increased in certain patient groups, including those with:
a prior history of haematological disorders;
previous treatment with chemotherapy (particularly alkylating agents and topoisomerase II inhibitors);
genetic disorders (e.g., inherited chromosomal fragility disorders, bone marrow failure syndromes, Li-Fraumeni syndrome, neurofibromatosis, chromosomal abnormalities [e.g., trisomy disorders]);
increasing age ( ≥65 years);
a history of smoking;
prior exposure to ionising radiation or benzene.
Patients with AML often present with a recent history of symptoms related to cytopenia, including fatigue, dizziness, palpitations, dysponea, fevers, infections, mucosal bleeding (e.g., from gums, nose, heavy menstrual bleeding), and petechial rash.
Some patients may report symptoms related to leukaemic infiltration, including bone pain (due to bone marrow infiltration); skin masses (e.g., myeloid sarcoma, due to skin infiltration); or neurological symptoms (e.g., headache, confusion, due to meningeal infiltration).[54][55]
Pulmonary symptoms (e.g., dyspnoea) and gastrointestinal symptoms (e.g., severe abdominal pain) may be present due to leukaemic infiltration or infection in the lungs and gastrointestinal tract, respectively.[56][57]
Physical examination
Findings may include signs of cytopenia (e.g., pallor, ecchymoses, and petechiae).
Signs of extramedullary leukaemic infiltration may be evident (e.g., hepatosplenomegaly, lymphadenopathy, gingival enlargement, skin masses, testicular masses).
Within the skin, leukaemia cutis infiltration may be present, and the presence of cutaneous ulcers (e.g., Sweet's syndrome or pyoderma gangrenosum) may indicate underlying malignancy.
Signs of oral/tooth infection (e.g., dental abscess), nasopharyngeal infection, pulmonary infection, or perianal infection may be present due to neutropenia.[58][59]
Rarely, an acute abdomen is noted on physical examination.
Initial laboratory tests
All patients with suspected AML should have the following baseline tests:[27][45]
Full blood count with differential
Peripheral blood smear
Comprehensive metabolic panel (serum electrolytes, renal and liver profiles, serum uric acid, and serum lactate dehydrogenase [LDH])
Coagulation panel (prothrombin time [PT], activated partial thromboplastin time [aPTT], fibrinogen, and D-dimers)
Laboratory findings
Most patients with AML (including those with acute promyelocytic leukaemia [APL], a subtype of AML) have anaemia, neutropenia, and/or thrombocytopenia, but blood count can vary greatly.
An elevated white blood cell (WBC) count >100 × 10⁹/L (>100,000/microlitre; hyperleukocytosis) occurs in approximately 5% to 20% of patients with AML, predisposing to complications such as tumour lysis syndrome (TLS), central nervous system (CNS) involvement, and leukostasis (symptomatic hyperleukocytosis; symptoms include respiratory distress and altered mental status).[3][4] These are medical emergencies and require immediate treatment. Despite the elevation in WBC count, many patients have severe neutropenia (absolute neutrophil count [ANC] <0.5 × 10⁹/L [<500/microlitre]), thus placing them at high risk for serious infections.
The AML, the blood film may show myeloid blasts characterised by Auer rods or Phi bodies. In APL, the blood film will typically show hypergranular promyelocytes with bilobed nuclei and bundles of Auer rods (as well as myeloid blasts). A variant of APL is characterised by hypogranular promyelocytes (absence of Auer rods), but is less common.[Figure caption and citation for the preceding image starts]: Peripheral blood film of a patient with acute myeloid leukaemia with maturation showing myeloid blasts with an Auer rodFrom the collection of Drs K. Raj and P. Mehta; used with patient consent [Citation ends].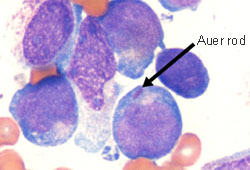 [Figure caption and citation for the preceding image starts]: Peripheral blood film of a patient with acute promyelocytic leukaemia showing hypergranular promyelocytes, some with bundles of Auer rodsFrom the collection of Drs K. Raj and P. Mehta; used with patient consent [Citation ends].
[Figure caption and citation for the preceding image starts]: Peripheral blood film of a patient with acute promyelocytic leukaemia showing hypergranular promyelocytes, some with bundles of Auer rodsFrom the collection of Drs K. Raj and P. Mehta; used with patient consent [Citation ends].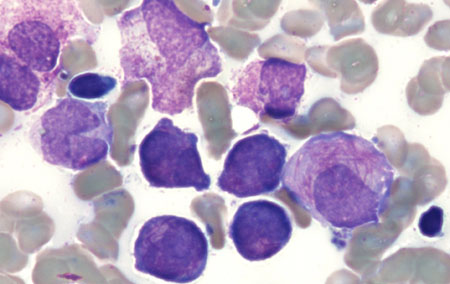 [Figure caption and citation for the preceding image starts]: Peripheral blood film of a patient with acute promyelocytic leukaemia showing hypergranular promyelocytes with bi-lobed nuclei and bundles of Auer rodsFrom the collection of Drs K. Raj and P. Mehta; used with patient consent [Citation ends].
[Figure caption and citation for the preceding image starts]: Peripheral blood film of a patient with acute promyelocytic leukaemia showing hypergranular promyelocytes with bi-lobed nuclei and bundles of Auer rodsFrom the collection of Drs K. Raj and P. Mehta; used with patient consent [Citation ends].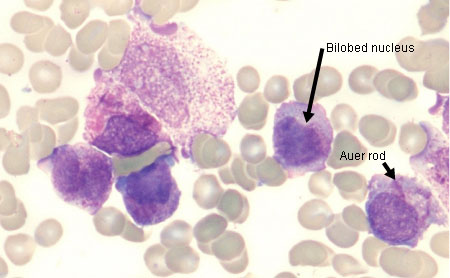
Hyperkalaemia, hyperphosphataemia, hyperuricaemia, hypocalcaemia, and elevated serum LDH may occur due to TLS, particularly during treatment and if WBC count (tumour burden) is high. This can lead to cardiac arrhythmias, seizures, acute kidney injury, and death, if untreated. TLS is an oncological emergency.[60] See Tumour lysis syndrome.
Hypercalcaemia may occur due to bony infiltration or ectopic release of a parathyroid hormone-like substance.
The coagulation tests PT and aPTT may be mildly prolonged with normal fibrinogen and D-dimer.
If coagulation tests are abnormal (prolonged PT and aPTT, decreased fibrinogen, and/or elevated D-dimer), disseminated intravascular coagulation (DIC) should be suspected and an urgent referral is warranted to commence treatment. Refer to the International Society on Thrombosis and Haemostasis (ISTH) scoring system for DIC.[61] DIC occurs most frequently in APL and is potentially life-threatening.[62].
Bone marrow evaluation
Diagnosis requires a bone marrow aspirate and trephine biopsy analysis. The following tests should be performed.[27][45]
Cytomorphology assessment: demonstrates bone marrow hypercellularity and infiltration by myeloid blasts (as well as hypergranular or hypogranular [less common] promyelocytes, in APL). Myeloid blast cells are negative for terminal deoxynucleotidyl transferase (TdT) and stain positive for myeloperoxidase.
Immunophenotyping using flow cytometry (on bone marrow aspirate) and immunohistochemistry (on core biopsy specimen): identifies cell surface and cytoplasmic markers of myeloid blasts (e.g., CD34, CD33) and establishes lineage.
If bone marrow specimens are inadequate or unattainable, then peripheral blood can be used for pathological assessment provided there are sufficient numbers of circulating blasts.[63]
Genetic testing
Cytogenetic analysis (karyotyping, fluorescence in situ hybridisation [FISH], or whole-genome sequencing) and molecular genetic testing (e.g., polymerase chain reaction [PCR], next-generation sequencing [NGS] assays) should be performed on bone marrow specimens (or peripheral blood if circulating blasts are present) to guide diagnosis, prognosis, risk stratification, and treatment.[27][45]
In AML, the following genetic abnormalities should be investigated due to their association with specific prognoses and treatment targets: RUNX1::RUNX1T1; CBFB::MYH11; MLLT3::KMT2A (or other KMT2A rearrangements); DEK::NUP214; BCR::ABL1; KAT6A::CREBBP; KIT; NPM1; FLT3 (ITD and TKD); IDH1; IDH2; CEBPA (basic leucine zipper [bZIP] domain); -5 or del(5q); -7; -17/abn(17p); GATA2; MECOM(EVI1); ASXL1; BCOR; EZH2; RUNX1; SF3B1; SRSF2; STAG2; U2AF1; ZRSR2; and TP53.[27][45] See Criteria.
APL is characterised by the PML::RARA fusion gene caused by a t(15;17)(q22;q12) balanced chromosomal rearrangement.[27][44]
Definitive diagnosis and classification
AML can be diagnosed and classified according to the latest World Health Organization (WHO) classification of haematolymphoid tumours (5th Edition, 2022) or the International Consensus Classification (ICC).[1][2] In both classifications, bone marrow evaluation, genetic testing, and predisposing factors (e.g., prior therapy, antecedent myeloid neoplasms, inherited genetic mutations or syndrome) are required to make a definitive AML diagnosis. However, the classifications differ in how specific subtypes of AML are categorised, and in the blast count threshold requirements for certain subtypes of AML.
The 5th edition of the WHO Classification does not require a blast threshold with the exception of AML with BCR::ABL1 fusion and AML with CEBPA mutation, which require ≥20% blasts for diagnosis.[1] The ICC classification, however, requires a blast count of ≥10% for diagnosing AML with defining genetic abnormalities (except for BCR::ABL1 fusion, where a blast count of ≥20% is required).[2]
See Classification section for further details.
Additional tests
Genetic testing for heritable haematologic malignancy predisposition syndromes (e.g., GATA2 deficiency syndrome, Shwachman-Diamond syndrome, dyskeratosis congenita) should be considered for certain patients, such as those <50 years of age, and those with a family history of a heritable haematologic disorder.[27][45] Findings may guide management.
CNS imaging (e.g., brain MRI or CT scan) should be performed to detect CNS bleeding, meningeal disease, or mass lesions in patients presenting with neurological signs or symptoms suggesting CNS involvement.[27][45] If CNS imaging does not identify CNS bleeding or a mass effect, and neurological signs and symptoms persist, lumbar puncture is recommended.[45] One dose of intrathecal chemotherapy (e.g., methotrexate or cytarabine, or a combination of both agents) can be considered at the time of diagnostic lumbar puncture.[45] Coagulopathy should be managed before lumbar puncture, particularly in patients with APL.
An FDG-PET/CT scan should be considered in patients with suspected extramedullary disease.[45]
Cardiac function assessment (echocardiogram or multigated acquisition scan) should be performed in patients:
with a history or symptoms of cardiac disease,
with prior exposure to cardiotoxic drugs or radiotherapy to the thorax, or
Cardiac findings may guide treatment.
Chest x-ray may be performed to identify pneumonia, mediastinal masses, pulmonary infiltrates, or cardiomegaly.
Human leukocyte antigen typing should be performed in all patients being considered for an allogeneic stem cell transplant.[45]
Use of this content is subject to our disclaimer