Recommendations
Urgent
This topic covers management of diabetic ketoacidosis (DKA) in adults.
Start intravenous fluids as soon as DKA is confirmed:[1][2][20][56][64]
If the initial systolic blood pressure (SBP) is <90 mmHg, give a fluid bolus of 500 mL of normal saline (0.9% sodium chloride) over 10-15 minutes via a large bore cannula.[2]
Give 1 L of normal saline over 1 hour if the initial SBP is >90 mmHg OR if SBP is >90 mmHg after fluid resuscitation.[2]
Give more cautious fluids and consider monitoring central venous pressure in patients who:[2]
are young (aged 18-25 years), elderly, or pregnant, or
have heart or kidney failure or other serious comorbidities.
Add potassium to the second litre of intravenous fluid if serum potassium is ≤5.0 mmol/L, using pre-mixed normal saline with potassium chloride.[1]
Start a fixed-rate intravenous insulin infusion (FRIII) according to local protocols; continue FRIII until DKA has resolved.[1][2][20][56][64]
Continue long-acting basal insulin (at the normal dose and time) if the patient is already taking this.[2]
Ensure intravenous fluids have already been started before giving a FRIII.
If potassium is <3.5 mmol/L, insulin therapy should be delayed or withheld until the potassium level is corrected to ≥3.5 mmol/L, in order to avoid life-threatening arrhythmias and respiratory muscle weakness.[1]
Ensure continuous cardiac monitoring and involve senior or critical care support if:[2][31]
There is persistent hypotension (SBP <90 mmHg) or oliguria (urine output <0.5 mL/kg/hour) despite intravenous fluids
Stupor and/or coma or Glasgow Coma Scale <12 [ Glasgow Coma Scale Opens in new window ]
Blood ketones (beta-hydroxybutyrate [BOHB]) >6 mmol/L
Venous bicarbonate <10 mmol/L
Venous pH <7.0
Potassium <3.5 mmol/L on admission
Oxygen saturations <92% on air
Pulse >100 bpm or <60 bpm
Anion gap >16 [ Anion Gap Opens in new window ]
The patient is pregnant or has heart or kidney failure or other serious comorbidities.
Identify and treat any precipitating acute illness.[2]
Key Recommendations
Management of diabetic ketoacidosis in adults
[Figure caption and citation for the preceding image starts]: Management of diabetic ketoacidosis 1. Intravenous fluidby BMJ Knowledge Centre [Citation ends].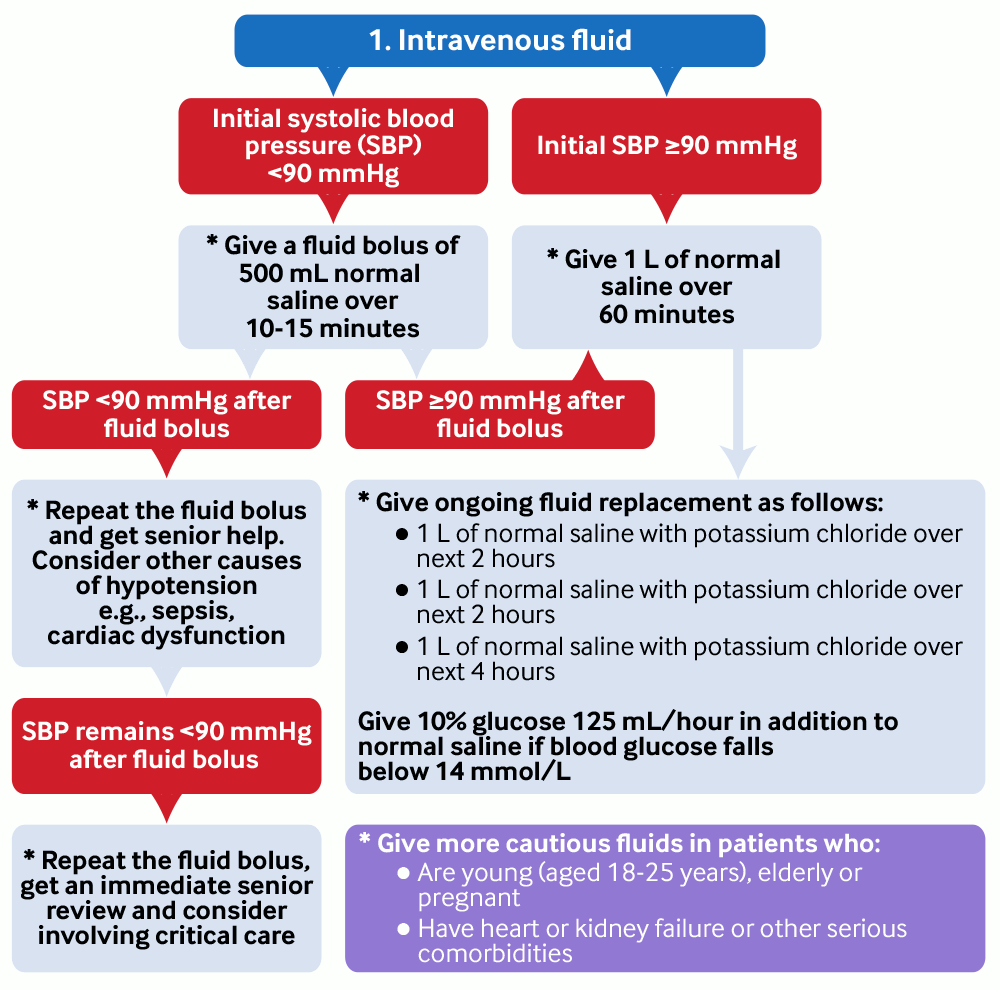 [Figure caption and citation for the preceding image starts]: Management of diabetic ketoacidosis 2. PotassiumCreated by BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Management of diabetic ketoacidosis 2. PotassiumCreated by BMJ Knowledge Centre [Citation ends]. [Figure caption and citation for the preceding image starts]: Management of diabetic ketoacidosis 3. InsulinCreated by BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Management of diabetic ketoacidosis 3. InsulinCreated by BMJ Knowledge Centre [Citation ends]. [Figure caption and citation for the preceding image starts]: Management of diabetic ketoacidosis 4. ResolutionCreated by BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Management of diabetic ketoacidosis 4. ResolutionCreated by BMJ Knowledge Centre [Citation ends].
Additional management during the first hour
Protect the airway.
Insert a nasogastric tube and aspirate if the patient is unresponsive to commands or is persistently vomiting.[2][56]
Insert a urinary catheter if there is incontinence or no urine is passed after 1 hour of starting treatment.[2][56]
Continue long-acting basal insulin if the patient is already taking this.[2]
Involve the specialist diabetes team as soon as possible and definitely within 24 hours.[2]
Ongoing management
Give ongoing fluid replacement once the first litre of fluid has been given.
If serum potassium is <3.5 mmol/L, involve senior or critical care support as a high dose of potassium needs to be given due to the severity of hypokalaemia.[2]
Potassium replacement is given using pre-mixed bags of normal saline containing 20-40 mmol/L of potassium chloride.[2]
To deliver a higher total dose, the infusion rate of this potassium-containing solution may be increased if the patient's fluid balance permits. If fluid volume is restricted, a more concentrated potassium infusion will be necessary.[2]
If serum potassium is 3.5 to 5.0 mmol/L,add 20-40 mmol/L potassium using pre-mixed normal saline (0.9% sodium chloride) with potassium chloride.[1][2][20][56] Consult local guidelines on potassium dosing.
If serum potassium is >5.0 mmol/L, avoid potassium-containing intravenous fluids and use normal saline instead.[1]
A typical fluid replacement regimen for a 70 kg patient with no other comorbidities is:[2][56]
Volume of normal saline (with potassium chloride as needed) |
|---|
1 litre over 2 hours |
1 litre over next 2 hours |
1 litre over next 4 hours |
1 litre over next 4 hours |
1 litre over next 6 hours |
Continue the FRIII until DKA has resolved. If the blood glucose falls to <14 mmol/L:[1][2][20][56][64]
Add 5% or 10% glucose. Give this concurrently with normal saline to correct dehydration.
Consider reducing the rate of intravenous insulin infusion to 0.05 units/kg/hour to avoid the risk of developing hypoglycaemia and hypokalaemia.
Monitor biochemical parameters to ensure these are improving.[2][56]
Measure venous bicarbonate, potassium, pH, blood glucose, and blood ketones as follows:
Ketones | Glucose | Bicarbonate | Potassium | pH | |
|---|---|---|---|---|---|
| 0 hours | ✓ | ✓ | ✓ | ✓ | ✓ |
| 1 hour | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2 hours | ✓ | ✓ | ✓ | ✓ | ✓ |
| 3 hours | ✓ | ✓ | |||
| 4 hours | ✓ | ✓ | ✓ | ✓ | ✓ |
| 5 hours | ✓ | ✓ | |||
| 6 hours | ✓ | ✓ | ✓ | ✓ | ✓ |
| 12 hours | ✓ | ✓ | ✓ | ✓ | ✓ |
Aim for a reduction in blood ketones of 0.5 mmol/L/hour if blood ketone measurement is available.[2]
Use venous bicarbonate or blood glucose if blood ketone measurement is unavailable. Aim for an increase in venous bicarbonate of 3 mmol/L/hour or a reduction in blood glucose of 3 mmol/L/hour.
Increase the FRIII according to local protocols if these targets are not met.
Monitor for cerebral and pulmonary oedema.[2]
Assess Glasgow Coma Scale hourly.
Order a chest x-ray if oxygen saturations fall and consider performing an arterial blood gas.
How to obtain an arterial blood sample from the radial artery.
How to perform a femoral artery puncture to collect a sample of arterial blood.
Resolution of DKA
By 24 hours, the ketonaemia and acidosis should have resolved. Seek senior or consultant input if this is not the case.[2] Resolution of DKA is defined as:[1]
Blood ketone level <0.6 mmol/L AND
Venous pH ≥7.3 or bicarbonate ≥18 mmol/L
Ideally, plasma glucose should also be <11.1 mmol/L. At this point, insulin dose can be decreased by 50%.[1]
Switch to subcutaneous insulin once DKA has resolved and the patient is eating and drinking. This should normally be done by the specialist diabetes team.[2]
Start subcutaneous insulin with a meal and continue the FRIII for 30-60 minutes after this.
Continue intravenous fluids and switch to a variable rate intravenous insulin infusion (VRIII) if DKA has resolved but the patient is not eating and drinking.[2]
Discharge
Ensure the patient has been reviewed by the diabetes specialist team before discharge and has follow-up arranged.[2]
Counsel patients about causes and early warning symptoms of DKA. Provide access to psychological support.
Treatment should aim to:[2][64]
Restore circulatory volume
Suppress ketogenesis
Correct electrolyte imbalance
Normalise blood glucose
Treat the precipitating cause and prevent complications.
Practical tip
This topic covers DKA in adults. Bear in mind that people aged 16 to 18 years may be managed by either a paediatric team or an adult medical team according to local arrangements. The Joint British Diabetes Societies for Inpatient Care (JBDS-IP) guideline recommends following paediatric guidelines if the patient is being managed by a paediatric team, and following adult guidance if they are being managed by an adult team.[2][3]
In the UK, the British Society for Paediatric Endocrinology and Diabetes publishes guidance for the management of DKA in children.[3]
Protect the airway.
Insert a nasogastric tube and aspirate if the patient is unresponsive to commands or persistently vomiting.[2][56]
Aspiration is a common complication of DKA due to gastroparesis.[56][132]
Insert a large bore cannula and start intravenous fluids as soon as DKA is confirmed.[2][64]
Seek immediate help from critical care if you are unable to get intravenous access.[2]
How to insert a peripheral venous cannula into the dorsum of the hand.
Give a fluid bolus of 500 mL normal saline (0.9% sodium chloride) over 10-15 minutes if the initial SBP is <90 mmHg.[2]
Repeat the fluid bolus if SBP remains <90 mmHg and seek senior help.[2]
Repeat the fluid bolus, get an immediate senior review and consider involving critical care if there is no improvement after the second fluid bolus.
Consider other causes of hypotension (e.g., sepsis, heart failure, acute myocardial infarction).[2]
Practical tip
Most patients with DKA and an initial SBP <90 mmHg require 500 to 1000 mL of fluid rapidly on arrival.[2]
More info: Management of mild/moderate DKA
Check your local protocols for recommendations on the management of mild DKA.
Globally, there are differences in management between countries reflecting access to healthcare resources, variations in diagnostic criteria, and the lack of published evidence to guide treatment.[20]
In UK practice, it is likely that all patients with DKA, regardless of severity, will be admitted to hospital. Notably, a 2024 consensus report by the American Diabetes Association (ADA), European Association for the Study of Diabetes(EASD), JBDS-IP, American Association of Clinical Endocrinology (AACE), and Diabetes Technology Society (DTS) advises that patients with uncomplicated mild or moderate DKA may be treated with subcutaneous rapid-acting insulin analogues rather than intravenous insulin. This recommendation is supported by evidence from randomised studies and meta-analyses, which have shown that administering subcutaneous rapid-acting insulin analogues every 1-2 hours is an effective alternative to intravenous short-acting insulin in this patient subgroup.[134][135][136] Treatment can be delivered in accident and emergency departments and step-down units without the need for intensive care unit care (ICU). This does not represent usual practice in the UK.
Give 1 L of normal saline over 1 hour once SBP >90 mmHg OR if initial SBP is >90 mmHg.[2]
Typical fluid deficits in DKA are:[2]
Water - 100 mL/kg
Sodium - 7 to 10 mmol/kg
Chloride - 3 to 5 mmol/kg
Potassium - 3 to 5 mmol/kg.
The aim of the first few litres of fluid is to:[2]
Correct any hypotension
Replenish the intravascular deficit
Correct any electrolyte disturbance.
Give more cautious intravenous fluids and consider monitoring central venous pressure in patients who:[2]
are young (aged 18-25 years), as rapid fluid replacement may increase the risk of cerebral oedema in these patients, or
are elderly or pregnant, or
have heart or kidney failure or other serious comorbidities.
Ultrasound-guided insertion of a non-tunnelled central venous catheter (CVC) into the right internal jugular vein using the Seldinger insertion technique.
Practical tip
Hartmann’s solution (Ringer’s lactate) is not normally used outside of critical care.[2] This is because it:
Contains 29 mmol/L of lactate, which can exacerbate the high lactate to pyruvate ratio in DKA and lead to adverse outcomes[147]
Raises the plasma lactate, which leads to more glucose being produced[147]
Contains 5 mmol/L of potassium, which can lead to fatal cardiac arrhythmias such as bradycardia or asystole if the patient has hyperkalaemia on arrival[147]
Contains bicarbonate, which can worsen the existing metabolic acidosis[147]
Is a hypotonic solution. This increases the risk of cerebral oedema in patients who are hyponatraemic on arrival.[147][148][149]
Debate: Normal saline versus balanced crystalloid solutions (e.g., Hartmann’s solution/Ringer’s lactate, Plasma-Lyte®) for resuscitation
There is a debate about the benefits of using normal saline (0.9% sodium chloride) over balanced crystalloid solutions in patients with DKA, as both have advantages and disadvantages.
In the UK, the JBDS-IP guideline recommends using normal saline for fluid resuscitation in patients with DKA on the general ward.[2] However, a 2024 consensus report by the ADA, EASD, JBDS-IP, AACE, and DTS supports the use of balanced crystalloid solutions (e.g., Ringer’s lactate, Plasma-Lyte®) as an alternative to normal saline in DKA management.[1]
The studies specifically discussed in the 2024 consensus report include:
A systematic review of four randomised controlled trials (RCTs; n=369 patients, 2 RCTs in adults) found that while the overall time to reach combined DKA endpoints was comparable between balanced crystalloid solutions and normal saline, balanced crystalloid solutions were generally associated with faster correction of pH.[150] However, the authors noted that findings were limited statistically due to the small number of studies and small patient population.
A post-hoc analysis of two cluster RCTs in adults suggested that balanced crystalloid may lead to faster resolution of DKA than normal saline, but not when given in a general ward (non-ICU) environment.[151]
A systematic review and meta-analysis of 8 RCTs (n=482 patients, 6 RCTs in adults) found that normal saline was associated with a longer time to DKA resolution (mean difference [MD] 3.5 hours), slightly longer hospital stay (MD 0.89 days), higher post-resuscitation serum chloride, and lower serum bicarbonate compared to balanced crystalloids.[152] The findings, based on low to moderate certainty evidence, suggest that balanced crystalloids may be preferable to saline for fluid resuscitation in DKA management.
Another meta-analysis of 3 RCTs in adults found that balanced crystalloid solutions were associated with a significantly faster resolution of DKA compared with normal saline, with a pooled hazard ratio of 1.46 (95% CI 1.10 to 1.94).[153] However, there was no statistically significant difference in time to resolution (MD -3.02 hours; 95% CI -6.78 to 0.74).
Normal saline for fluid resuscitation in patients with DKA
Advantages:
Normal saline is readily available on general wards and clinicians are experienced with this product.[2]
Normal saline is available with pre-mixed potassium so it complies with the UK National Patient Safety Agency (NPSA) recommendations. The NPSA states that commercially prepared ready to use diluted solutions containing potassium should be used wherever possible to reduce the risk of misadministration of potassium, so normal saline is recommended in the UK.[2][154]
It is important to avoid the need to add concentrated potassium to resuscitation fluids, as there is a risk of death from misadministration of concentrated potassium.[155]
Disadvantages:
Hyperchloraemic metabolic acidosis is a possible complication, due to the large volume of sodium chloride required for fluid resuscitation in DKA.[2]
Hyperchloraemic metabolic acidosis may result in a delay to resolution of the acidosis because it can cause arterial vasoconstriction in the kidneys and subsequent oliguria.[2]
Hartmann’s solution (Ringer’s lactate) for fluid resuscitation in patients with DKA
Advantages:
Hartmann’s solution has a minimal tendency to cause hyperchloraemic metabolic acidosis, due to the lower chloride content than normal saline.[2]
Disadvantages:
In general, doctors in the UK are less familiar using Hartmann’s solution clinically and it is not as readily available in clinical areas.[2]
Hartmann’s solution contains potassium, which could be harmful in early DKA.[147]
However, it also does not contain enough potassium if used alone once the potassium levels begin to fall. It is also not commercially available with pre-mixed potassium and therefore it does not comply with UK NPSA recommendations.[2]
Hartmann’s solution can increase the lactate to pyruvate ratio, which is already raised in DKA, worsen acidosis due to its bicarbonate content, and worsen cerebral oedema as it is hypotonic.[147]
If serum potassium is 3.5 to 5.0 mmol/L, add 20-40 mmol/L of potassium to the second litre of intravenous fluid using pre-mixed normal saline with potassium chloride, in line with local guidelines on potassium dosing.[1][2][56] Then continue intravenous fluid therapy with potassium replacement according to the potassium level on venous blood gas as follows:[1][2][56]
Potassium level (mmol/L) | Potassium replacement (mmol/L of infusion solution) |
|---|---|
<3.5 | Involve senior or critical care support as additional potassium needs to be given |
3.5 to 5.0 | 20-40 |
>5.0 | None |
Use normal saline with pre-mixed potassium chloride as the default fluid for resuscitation in DKA.
Manually adding potassium to intravenous fluids in general clinical areas is unsafe as this can result in accidental overdose of potassium, which can be fatal.[154]
Hyperkalaemia and hypokalaemia are life-threatening complications and common in DKA.[2][20]
These can precipitate life-threatening cardiac arrhythmias.
Serum potassium is often high on admission (although total body potassium is low).
Start a FRIII according to local protocols.[2][20][64][56]
Ensure intravenous fluids have been started before giving a FRIII.
If potassium is <3.5 mmol/L, insulin therapy should be delayed or withheld until the potassium level increases to ≥3.5 mmol/L, in order to avoid life-threatening arrhythmias and respiratory muscle weakness.[1]
Seek advice from the diabetes specialist team if >15 units/hour of insulin are required.[2][68]
Practical tip
Only give an intramuscular bolus of insulin if there is a delay in setting up a FRIII.[2]
Write out ‘units’ when prescribing insulin. Never use abbreviations such as ‘U’ or ‘IU’.[2]
Estimate the patient’s weight if necessary.[2]
If the patient is pregnant, use the current pregnancy weight and call for immediate senior obstetric help.[2]
Avoid rapid correction of hyperglycaemia as this increases the risk of cerebral oedema.[79]
Ensure continuous cardiac monitoring and seek senior or critical care support if:[2][31]
There is persistent hypotension (SBP <90 mmHg) or oliguria (urine output <0.5 mL/kg/hour) despite intravenous fluids
Stupor and/or coma or Glasgow Coma Scale <12 [ Glasgow Coma Scale Opens in new window ]
Blood ketones (beta-hydroxybutyrate [BOHB]) >6 mmol/L
Venous bicarbonate <10 mmol/L
Venous pH <7.0
Potassium <3.5 mmol/L on admission
Oxygen saturations <92% on air
Pulse >100 bpm or <60 bpm
Anion gap >16 [ Anion Gap Opens in new window ]
The patient is pregnant or has heart or kidney failure or other serious comorbidities.
Consider giving bicarbonate only if venous pH <7.0 and after discussion with a senior consultant.[1] Monitor the patient in a critical care environment.[156]
Bicarbonate has been associated with the development of cerebral oedema.[156]
Evidence: Intravenous bicarbonate
The UK JBDS-IP guideline on management of DKA does not recommend routine use of intravenous bicarbonate for DKA in adults, stating that acidosis will resolve with adequate fluid and insulin therapy.[2]
The JBDS-IP guideline cites a systematic review that reported data from 44 studies of bicarbonate treatment for severe acidaemia in patients with DKA, including three randomised controlled trials (RCTs) in adults (total number: 73 adults).[157]
Two of the included RCTs in adults showed transient improvement in metabolic acidosis with bicarbonate within the initial 2 hours, but no evidence of improved glycaemic control or clinical efficacy [157]
The guideline found no studies reporting on cerebral oedema in adults or on patients with an admission pH <6.85 [157]
Retrospective studies in children receiving bicarbonate for DKA have found an increased risk of cerebral oedema and prolonged hospitalisation [157]
Routine administration of bicarbonate is also not recommended in a 2024 consensus report by the ADA, EASD, JBDS-IP, AACE, and DTS.[1]
Seek senior advice if your patient has severe acidosis, or if bicarbonate treatment is being considered.
Practical tip
Do not routinely replace phosphate.[2]
Discuss severe hypomagnesaemia with a senior; magnesium may need to be replaced but there is no guidance for cut-off values.
Insert a urinary catheter if there is incontinence or no urine is passed after 1 hour of starting treatment.[2][56]
Give a dose of long-acting insulin to prevent rebound hyperglycaemia when DKA has resolved and the FRIII is stopped.[2]
Continue long-acting basal insulin if the patient is already taking this.
If this is the first presentation of diabetes, start a long-acting basal insulin as soon as possible.
Practical tip
Never omit insulin in any patient with type 1 diabetes as this can precipitate DKA.[20]
In a UK survey, more than 7% of DKA episodes occurred in hospitalised patients.[158] It is often wrongly assumed that patients aged over 50 years have type 2 diabetes and can tolerate periods of insulin omission when admitted to hospital.
Continuing long-acting insulin during DKA also prevents rebound hyperglycaemia when the FRIII is stopped.[2]
Involve the specialist diabetes team as soon as possible and definitely within 24 hours.[2]
The specialist diabetes team should also be involved in the assessment of the cause of DKA.
It is unsafe to manage DKA without the specialist diabetes team and could compromise patient care.[2]
Identify and treat any precipitating acute illness.[2]
Consider thromboprophylaxis with low molecular weight heparin in all patients, unless it is contraindicated.[2] Consult local guidelines. See Venous thromboembolism (VTE) prophylaxis.
Ensure effective handover of patients with DKA.
Give relevant details on the clinical and biochemical progress of the patient and the plan for further management.
Practical tip
Handover is a common source of error in managing DKA.
Management at 1 to 6 hours
Review the patient hourly to ensure clinical and biochemical improvement and continue the FRIII.[2][56]
Order hourly blood glucose and hourly blood ketones.
Perform a venous blood gas for pH, bicarbonate, and potassium at 60 minutes, 2 hours, and 2 hourly thereafter.
Aim for a reduction in blood ketones of 0.5 mmol/L/hour if blood ketone measurement is available.
Use venous bicarbonate or blood glucose measurement if blood ketone measurement is not available.
Aim for an increase in venous bicarbonate of 3 mmol/L/hour or a reduction in blood glucose of 3 mmol/L/hour.
If the target rates for blood ketones, blood glucose, and venous bicarbonate are not achieved:[2]
Check the insulin infusion pump is working and connected and that the correct insulin residual volume is present (to check for pump malfunction)
Increase the insulin infusion according to local protocols (if there is no insulin pump malfunction) until the target rates for ketones, glucose, and bicarbonate are achieved.
Practical tip
Monitor all patients with DKA closely:
DKA is complicated to manage and needs close monitoring and timely treatment modifications.[20]
Use of standardised paper-based and computerised guidelines and protocols for management of DKA has been shown to decrease the time to anion gap closure, reduce length of stay in hospital, and minimise complications. However, treatment protocols are often not followed.[20]
It is important to recognise that, despite their usefulness, guidelines and protocols should not replace sound clinical judgement.[20]
Continue intravenous fluid replacement.
A typical regimen for a 70 kg adult with no other comorbidities is:[2][20][56]
Volume of normal saline (with potassium chloride as needed) |
|---|
1 litre over 2 hours |
1 litre over next 2 hours |
1 litre over next 4 hours |
1 litre over next 4 hours |
1 litre over next 6 hours |
Give more cautious intravenous fluids and consider monitoring central venous pressure in patients who:[2]
are young (aged 18-25 years) as rapid fluid replacement may increase the risk of cerebral oedema in these patients, or
are elderly or pregnant, or
have heart or kidney failure or other serious comorbidities.
Ultrasound-guided insertion of a non-tunnelled central venous catheter (CVC) into the right internal jugular vein using the Seldinger insertion technique.
Maintain an accurate fluid balance chart.[2]
Aim for a minimum urine output of 0.5 mL/kg/hour.
Maintain the potassium level between 4.0 and 5.0 mmol/L. Replace potassium via intravenous fluids if serum potassium is ≤5.0 mmol/L, using pre-mixed normal saline with potassium chloride.[1][2] Adjust potassium replacement as follows:[1][2]
Potassium level (mmol/L) | Potassium replacement (mmol/L of infusion solution) |
|---|---|
<3.5 | Involve senior or critical care support as additional potassium needs to be given |
3.5 to 5 | 20-40 |
>5 | None |
Consult local guidelines on potassium dosing.
If the glucose level falls to <14 mmol/L:[1][2][64]
Give 5% or 10% glucose in addition to normal saline and continue until the patient is eating and drinking normally
Consider reducing the rate of intravenous insulin infusion to 0.05 units/kg/hour to avoid the risk of developing hypoglycaemia and hypokalaemia.
Practical tip
A common mistake is allowing hypoglycaemia to develop, as blood glucose levels can fall quickly during the correction of ketoacidosis.[2]
This may lead to a rebound ketosis (driven by counter-regulatory hormones) and lengthen the duration of treatment.
Severe hypoglycaemia is associated with cardiac arrhythmias, acute brain injury, and death.
It is important to give glucose and sodium chloride solutions concurrently if the glucose level falls to <14 mmol/L.[2] Also consider reduction of the insulin infusion rate.[2]
Evidence: Reduction of insulin rate when glucose concentrations drop to <14 mmol/L
In people with DKA, reducing the insulin rate once blood glucose falls below 14 mmol/L may help reduce the risk of hypoglycaemia and hyperkalaemia.
The JBDS-IP guideline on DKA management specifically addressed concerns around hypoglycaemia and hypokalaemia, which had been identified as common complications in a UK national survey, despite widespread implementation of earlier JBDS-IP recommendations.[2][158]
The main cause was the use of insulin.
Although there is an absence of trial evidence in adults with DKA, the panel noted that several other adult guidelines suggest reducing the rate of intravenous insulin infusion as blood glucose levels fall.[2]
One RCT in children with DKA (n=50) found that a lower rate of insulin infusion (0.05 units/kg/hour compared with 0.1 units/kg/hour) did not significantly delay resolution of acidosis, but was associated with lower rates of hypokalaemia (20% vs. 48%) and hypoglycaemia (4% vs. 20%).[159][160]
A second paediatric RCT (n=60) reported similar findings.[161]
Based on recommendations from other guidelines and indirect evidence from paediatric studies, the JBDS-IP panel recommended that in adults with DKA, the insulin infusion rate should be reduced to 0.05 units/kg/hour when blood glucose falls below 14 mmol/L.[2] This recommendation is further supported by a 2024 consensus report by the ADA, EASD, JBDS-IP, AACE, and DTS.[1]
Monitor for complications regularly throughout treatment of DKA.[2][56]
Assess Glasgow Coma Scale hourly to monitor for cerebral oedema.[2]
Monitor vital signs closely according to local protocols.
Request a chest x-ray if oxygen saturations fall as this may be a sign of pulmonary oedema. Consider performing an arterial blood gas.
Pulmonary oedema and acute respiratory distress syndrome (ARDS) are rare but significant complications of treatment for DKA and present with fluid overload and low oxygen saturations.[163] They occur when excess fluid is given, even in patients with normal cardiac function.
Look for an increased alveolar to oxygen gradient (AaO2) and auscultate for lung crepitations.
Pulmonary oedema and ARDS are more common in patients who are severely dehydrated or with higher glucose levels on arrival.
How to obtain an arterial blood sample from the radial artery.
How to perform a femoral artery puncture to collect a sample of arterial blood.
Practical tip
Other features of cerebral oedema are recurrent vomiting, incontinence, irritability, abnormal respirations, and cranial nerve dysfunction. These usually occur several hours after starting treatment.[2][79]
The exact cause of cerebral oedema is unknown. It occurs most commonly in children and adolescents, and is rare over the age of 28. It is the most common cause of mortality in DKA.[2][63][79]
Consider thromboprophylaxis with low molecular weight heparin in all patients, unless it is contraindicated.[2][56] Check your local protocol.
Management at 6 to 12 hours
Continue intravenous fluids, potassium correction, and FRIII.[2][20] Seek senior advice if clinical and biochemical markers are not improving.
Check ketones, blood glucose, venous pH, bicarbonate, and potassium at 6 hours.
Assess for resolution of DKA. This is defined as:[1]
Blood ketone level <0.6 mmol/L AND
Venous pH ≥7.3 or bicarbonate ≥18 mmol/L
Ideally, plasma glucose should also be <11.1 mmol/L. At this point, insulin dose can be decreased by 50%.[1]
Practical tip
Do not rely on bicarbonate alone or the anion gap to assess for resolution of DKA.[1][2][79]
A hyperchloraemic acidosis typically persists secondary to high volumes of normal saline. This lowers the bicarbonate and leads to difficulty in assessing whether ketosis has resolved.
The hyperchloraemic acidosis may cause renal vasoconstriction and oliguria.
Around one third of patients presenting with DKA have a mixed acid-base disorder due to hyperglycaemia-induced osmotic diuresis and natriuresis, nausea and vomiting, and hyperventilation (Kussmaul respiration).
Do not rely on urinary ketone clearance to assess resolution of DKA.[2]
Urine ketones will still be present when DKA has resolved.
Management at 12 to 24 hours
Check venous pH, bicarbonate, potassium, ketones, and glucose at 12 hours. Ensure DKA has resolved within 24 hours.[2][56]
Request senior or specialist input if DKA has not resolved within 24 hours.[2]
It is unusual for patients not to respond to treatment so it is important to identify and treat the cause of DKA.
Continue the FRIII and intravenous fluids.
Monitor blood ketones and glucose hourly, and pH, potassium, and bicarbonate every 2 hours to ensure they fall at the specified target rates.
Start regular subcutaneous insulin when DKA has resolved and the patient is eating and drinking. This should normally be done by the diabetes specialist team and given with a meal.[2][79]
Switch to subcutaneous insulin from intravenous insulin in the morning if possible, as most hospitals have better staffing during daytime, should DKA recur.[132]
Continue intravenous insulin for 30-60 minutes after administering subcutaneous insulin to prevent relapse of DKA.[2]
It is a common error to stop intravenous insulin either too early or before the timing and doses of subcutaneous insulin have been sorted out.[20]
If the patient was on basal bolus insulin:[2]
This should have been continued if they were taking a long-acting insulin analogue[20]
Restart their normal short-acting subcutaneous insulin at the next meal
In general, restart the patient’s previous insulin regimen if their most recent HbA1c shows an acceptable level of control l (i.e., HbA1c 64 mmol/mol)[2]
Do not stop the intravenous insulin if the long-acting insulin has been stopped in error until a form of background insulin has been given.[2]
Give half the usual daily dose of basal insulin (using insulin isophane) in the morning if the basal analogue was normally taken once daily in the evening and the intention is to convert to subcutaneous insulin in the morning.
Check the blood ketone and glucose levels regularly.
If the patient was on twice daily fixed-mix insulin:[2]
Re-introduce the subcutaneous insulin before breakfast or before the evening meal. Do not change at any other time.
If the patient was on continuous subcutaneous insulin infusion (CSII):[2]
Restart the CSII at the normal basal rate
Continue the intravenous insulin infusion until the meal bolus has been given
Do not restart CSII at bedtime.
Estimate the total daily dose (TDD) of insulin for patients who were not previously taking insulin.[2]
Take into account the patient’s sensitivity to insulin, degree of glycaemic control, insulin resistance, and age.[2]
A weight-based formula may be considered, using 0.5 to 0.6 units/kg/day in insulin-naive patients, bearing in mind that body composition and/or insulin resistance may have an impact on this estimate.[1] For people with risk factors for hypoglycaemia, including kidney failure or frailty, a calculation using approximately 0.3 units/kg/day may be more appropriate.[1] Use 0.75 units/kg for people thought to be more insulin resistant (e.g., adolescents, people with obesity).[2]
If using a basal bolus regimen:
Give 50% of the TDD with the evening meal as long-acting insulin and divide the remaining dose equally between pre-breakfast, pre-lunch, and pre-evening meal.
Give the first dose of fast acting subcutaneous insulin preferably before breakfast.
Only give the first dose before the evening meal if monitoring is in place.
Never convert to a subcutaneous regimen at bed time.
If using a twice daily pre-mixed regimen:
Give two-thirds of the TDD at breakfast and give the remaining third with the evening meal.
Practical tip
Use a basal bolus regimen for most patients, especially the young and fit.
Consider a twice daily pre-mixed regimen for older patients as they may not be able to manage a basal bolus regimen.
Continue intravenous fluids if the patient is not eating and drinking.[2][20]
Start a variable rate intravenous insulin infusion (VRIII) for these patients if DKA has resolved.
Measure blood glucose regularly.
Counsel patients about the precipitating cause and early warning symptoms of DKA. Consider:[2][20]
Review of their usual glycaemic control
Review of their injection technique, blood glucose monitoring, equipment, and injection sites
Prevention of recurrence (e.g., provide written ‘sick day rules’)
Checking the patient’s insulin prior to reuse (this may be expired or denatured)
Assessing the need for provision of handheld ketone meters for use at home
Providing a contact number on how to contact the diabetes specialist team out of hours
Providing a written care plan which allows the patient to have an active role in their diabetes management, with a copy of this sent to their GP.
Be aware that in the UK, all patients with type 1 diabetes mellitus should be offered continuous glucose monitoring, based on discussion of a range of factors, including whether erratic blood glucose is affecting their quality of life.[56]
Ensure patients have appropriate follow-up with the diabetes specialist team and access to psychological support.[2]
Ultrasound-guided insertion of a non-tunnelled central venous catheter (CVC) into the right internal jugular vein using the Seldinger insertion technique.
How to obtain an arterial blood sample from the radial artery.
How to perform a femoral artery puncture to collect a sample of arterial blood.
How to insert a peripheral venous cannula into the dorsum of the hand.
Use of this content is subject to our disclaimer