Aetiology
Neural tube defects result from a complex interplay between genetic and environmental factors.[29] This is demonstrated by the effectiveness of folate for prevention and by higher rates of neural tube defects among certain racial and ethnic populations. For example, in the US, Hispanic immigrant parents have a more than 3-fold increased risk of having a pregnancy affected by a neural tube defect compared with the general population.[30] In contrast, US-born Hispanic parents have only a 2-fold increased risk of having a pregnancy affected by anencephaly and have rates of spina bifida that are similar to those for non-Hispanic white people.[31] Women who already have a child with spina bifida, or who have had a pregnancy affected by a neural tube defect, or who have spina bifida themselves, have a 30-fold increased risk of having a baby with spina bifida compared with the general population.[2][32] Furthermore, the risk of neural tube defects among children born to siblings of individuals affected by spina bifida is double that of the general population.[7]
Trisomy 18, trisomy 13, and 22q deletion syndrome (also known as velocardiofacial syndrome) are chromosome disorders that are commonly associated with neural tube defects.[2][32][33]
Other known risk factors include maternal obesity, maternal diabetes, and antenatal exposure to certain drugs such as valproic acid (and its derivatives), carbamazepine, isotretinoin, and methotrexate.[25][34][35][36] Low socioeconomic status also contributes to the risk of neural tube defects, even after adjusting for maternal nutritional status.[37] In addition, maternal core body temperature elevated by 2° F or C during the first trimester (due either to fever or to the use of saunas or hot tubs) has been shown to increase the risk of neural tube defects.[38]
The single most clinically significant protective factor known to reduce the incidence of spina bifida is maternal folate consumption. An association between vitamin deficiencies and neural tube defects was first observed in 1976.[39] Subsequent studies found that folic acid supplementation reduced the risk of spina bifida recurrence among couples who had previously conceived a child with spina bifida.[40][41] Observational studies of folate fortification programmes have documented conclusively that folate reduces the incidence of neural tube defects.[18] It is estimated that there would be a 50% to 70% reduction of neural tube defects if maternal folate intake were optimised according to public health recommendations.[42] Inadequate maternal vitamin B12 intake has also been associated with an increased risk of neural tube defects.[43]
Neural tube defects are believed to result from enzymatic abnormalities of metabolic pathways that require folate and its metabolites tetrahydrofolate and 5-methyltetrahydrofolate.[29] The folate-dependent pathways are involved with single carbon transfers required for the synthesis of nucleotides and in methylation reactions, including the remethylation of homocysteine to methionine. Several candidate genes have been studied, including those encoding folate receptors, 5,10 methylene tetrahydrofolate reductase (MTHFR), methionine synthase, and methionine synthase reductase, which are also dependent upon the availability of vitamin B12. Genes involved with folate-homocysteine metabolism have also been implicated.[29][44]
Pathophysiology
The malformation that causes neural tube defects occurs during embryogenesis, typically no later than 26 days after fertilisation.
Two different processes lead to formation of the central nervous system (CNS). The first is primary neurulation, which refers to the invagination of the neural plate into the neural tube, and subsequently the embryonic brain and spinal cord. Secondary neurulation refers to the formation of the lower spinal cord, which gives rise to the lumbar and sacral elements. Any disruption that occurs when the neural plate begins its first fold and fuses to form the neural tube (days 17-23) can cause craniorachischisis, the most severe form of neural tube defect. Closure of the rostral neuropore occurs between days 23 and 26. Disruption during this phase of embryogenesis results in anencephaly. Myelomeningocele results when the closure of the caudal neuropore is disrupted during days 26 to 30.[2]
In patients with open spina bifida, all parts of the nervous system, including the brain, are affected. It is hypothesised that the open lesion on the spine causes the intraspinal pressure to be lower than the intracranial pressure, leading to hind brain herniation and the Chiari malformation, which consequently alters the flow of cerebrospinal fluid, and results in hydrocephalus.[45] An alternate theory is that spina bifida is a manifestation of progressive hydrocephalus in the fetus because of a mesodermal growth insufficiency that influences both neural tube closure and CNS pressure, leading to dysraphism.[46] Neuro-epithelial or ependymal denudation in the telencephalon and the aqueduct has been documented in the fetal brain prior to the development of Chiari II malformation and hydrocephalus. Because denuded areas cannot re-establish cell function, it has also been hypothesised that abnormalities in the cells lining the ventricles of the brain induce permanent cerebral pathology. Finally, diffusion tensor tractography has documented abnormal white matter development in the association pathways of children with spina bifida, which may explain the specific learning problems, executive dysfunction, and attention deficits that often accompany hydrocephalus.[47] Brain anomalies associated with myelomeningocele include agenesis of the corpus callosum, lobar agenesis, polymicrogyria, grey matter heterotopia, and intracranial cysts and lipomas.
Classification
Neural tube defects of the spinal cord[1][2]
Open spina bifida (not covered by skin)
Myelomeningocele: herniation of both meninges and spinal cord. Associated with hydrocephalus and Chiari II malformation. [Figure caption and citation for the preceding image starts]: Neonate with myelomeningoceleFrom the collection of Dr Greg Liptak; used with permission [Citation ends].
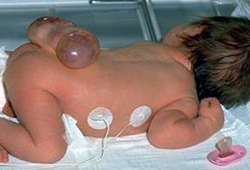 [Figure caption and citation for the preceding image starts]: MyelomeningoceleFrom the collection of Dr Greg Liptak; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: MyelomeningoceleFrom the collection of Dr Greg Liptak; used with permission [Citation ends].
Myeloschisis: herniation of meninges and spinal elements. Characterised by flattened, plate-like mass of disorganised neural tissue.
Meningocele: herniation of the meninges without involvement of spinal elements. [Figure caption and citation for the preceding image starts]: MeningoceleFrom the collection of Dr Zulma Tovar-Spinoza; used with permission [Citation ends].
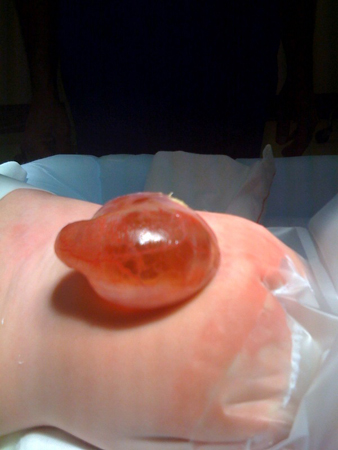
Closed spina bifida (covered by skin)
Spine defects that are associated with a visible abnormality of the back such as asymmetrical gluteal fold or dimple, haemangioma, hairy patch, or other cutaneous markings. These lesions are also referred to as occult spinal dysraphism.
Myelocystocele: multi-cystic lesion continuous with the central canal of the spinal cord; associated with exstrophy of the cloaca.
Lipomyelomeningocele, lipomeningocele, lipomyelocystocele: closed defects associated with fatty tumours.
Congenital tethered spinal cord: lower end of the spinal cord has abnormal attachment to vertebral column or subcutaneous tissues. Often associated with low-lying conus (below L2-L3 interspace).
Fatty filum terminale: fatty tumour at the end of the spinal cord.
Thickened filum terminale: thickening at the end of the spinal cord.
Diastematomyelia: fibrous bands, bone, or cartilage in the spinal canal; often associated with split spine.
Syringomyelia: dilation of the central canal of the spinal cord. Can result in scoliosis, upper extremity weakness, and numbness; associated with tethered cord and shunt malfunction.
Dermal sinus tract: connection between the spinal canal and the skin.
Spina bifida occulta (skin covered with no visible abnormalities of the back)
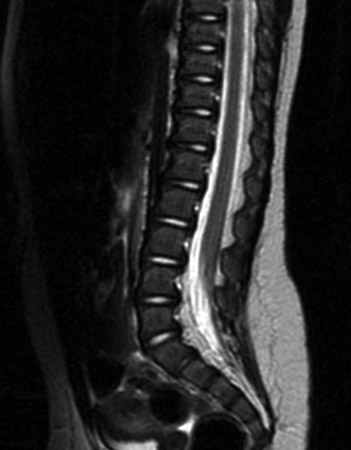
Vertebral fusion defect only, without spine involvement and of no clinical significance. Incidental radiological finding present in 10% to 15% of the population. [Figure caption and citation for the preceding image starts]: Spine MRI scan showing tethered cordFrom the collection of Dr Nienke P. Dosa; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: MyelomeningocystoceleFrom the collection of Dr Zulma Tovar-Spinoza; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: MyelomeningocystoceleFrom the collection of Dr Zulma Tovar-Spinoza; used with permission [Citation ends].
Neural tube defects of the brain[1][2]
Anencephaly: total or partial absence of brain and calvarium. Many fetuses with anencephaly are spontaneously aborted; few survive infancy.
Iniencephaly: abnormal or incomplete closure of the neural tube in the occipital region, which results in severe retroflexion of neck and trunk; incompatible with life.
Encephalocele: defect in calvarium with protrusion of brain, most often in occipital region. Occipital encephaloceles are associated with intellectual disability, hydrocephalus, spasticity, and seizures. Patients with frontal encephaloceles have better developmental outcomes.
Craniomeningocele: defect in calvarium with protrusion of meninges.
Neural tube defects of both the brain and spinal cord[1][2]
Craniorachischisis: anencephaly accompanied by contiguous bony defect of spine; incompatible with life.
Use of this content is subject to our disclaimer