Investigations
1st investigations to order
stool ova and parasites (O&P) examination
Test
The sensitivity of stool microscopy is dependent on the experience of the laboratory performing the test. In experienced laboratories, the sensitivity of three positive specimens is 50% to 75%.[5][11][14]
Agar plate cultures of faeces are more sensitive, but not routinely employed by microbiology laboratories.[34]
Result
strongyloides larvae seen on stool examination
FBC with differential
Test
Sensitivity is 80% to 85%.[11][15]
Asymptomatic eosinophilia is present in 10% to 15% of newly arriving refugees.[35]
Eosinophilia is frequently absent (40%) when a patient is receiving corticosteroids and presenting with hyperinfection.[20][21]
Eosinophilia is often greater in children with infection than in adults.
Result
>0.4 × 10⁹ eosinophils/L (>400 eosinophils/microlitre) or >5% relative eosinophilia
therapeutic trial with ivermectin (in specific situations)
Test
Empirical ivermectin is recommended for people immigrating from endemic areas requiring urgent corticosteroids for acute conditions (such as asthma), especially in the presence of eosinophilia.
At 6-12 months post-ivermectin therapy, two-thirds of patients will either have a negative serology or quantitatively the titre is decreased by 40% or more. At follow-up at 6 months, eosinophilia will have resolved in successfully treated people.[11]
Result
at follow-up at 6 months' post-treatment, eosinophilia will have resolved in successfully treated people
Investigations to consider
sputum O&P examination
Test
In hyperinfection, larvae may be detectable on sputum O&P.[11]
Result
strongyloides larvae seen
clinical sample (non-stool or sputum) O&P examination
Test
Disseminated strongyloides infection is definitively diagnosed with the identification of strongyloides larvae in another clinical specimen (not stool or sputum) or biopsy from a source other than the routine strongyloides life cycle of pulmonary and GI tracts.
Result
strongyloides larvae seen
strongyloides IgG serology
Test
If stool specimens are negative in a migrant with eosinophilia, from an endemic area, serology should be considered.[15]
Serology testing by enzyme-immuno assay (EIA), which has 96% sensitivity and 98% specificity, is obtainable from reference laboratories upon request.[31]
Result
positive serology
tissue biopsy
Test
This may be an incidental finding on a dermatological biopsy.[Figure caption and citation for the preceding image starts]: Strongyloides stercoralis larva in tissueFrom the collection of David Boulware, University of Minnesota; used with permission [Citation ends].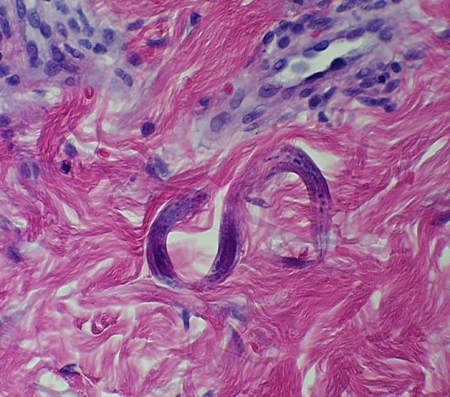
Disseminated strongyloides infection may be diagnosed with the identification of strongyloides larvae in a biopsy from a source other than the routine strongyloides life cycle sites of pulmonary and gastrointestinal tracts.
Result
rhabditiform larvae visualised in tissue biopsy
Emerging tests
polymerase chain reaction (PCR)
Test
PCR is emerging as a molecular diagnostic tool for diagnosing strongyloides infection. PCR assays have been shown to have greater sensitivity than traditional O&P examination.[32] Commercial PCR assays for strongyloides infection are currently in development.
Result
DNA detection
Use of this content is subject to our disclaimer