Approach
Neutropenia is usually diagnosed when a patient presents with fever or recurrent bacterial infections. It may also be diagnosed on routine laboratory work or when a patient is being evaluated for another disorder. If detected, it should be confirmed by repeat testing with a full blood count (FBC), differential, and manual examination of the peripheral blood smear.[54][55]
The diagnostic approach differs between adults and children. In adults, the most likely causes are infection, drug-induced neutropenia, or primary acquired bone marrow disease. In children, the most likely cause is an immune neutropenia. Infection and drugs remain common causes. A stepwise approach is required in children to detect congenital disease.
General history and examination in adults
Adults usually present with fever, malaise, infection, or mouth sores. Neutropenia is most frequently acquired from infectious, drug-induced, malignant, or autoimmune causes. There is usually no prior history or family history of neutropenia. Immune neutropenia is rare in adults and does not increase the risk of infection. Pseudoneutropenia should only be considered as a diagnosis in an asymptomatic patient with a normal bone marrow biopsy.
Key features of the history in adults include the following.
Infection: characteristic risk factors and symptoms of known infectious causes and sequelae should be sought.
Drug and treatment history: the use of any medication known to cause neutropenia should prompt suspicion of an adverse drug reaction.[1][12][54][56] Anticoagulants can also produce pseudoneutropenia. Neutropenia is an expected adverse effect of chemotherapy or radiotherapy.[57] Associated symptoms of anaemia (fatigue, dyspnoea, and dizziness), thrombocytopenia (bleeding and easy bruising), swollen lymph nodes, night sweats, or weight loss should prompt consideration of haematological malignancy. Symptoms of anaemia and thrombocytopenia may also indicate acquired aplastic anaemia or myelodysplastic syndrome.
Nutritional history: inadequate dietary intake may lead to vitamin B12, folate, or copper deficiency. This is particularly common in people with alcohol-use disorder.
Medical history: chronic inflammatory disease may be present (rheumatoid arthritis, systemic lupus erythematosus [SLE], Felty's syndrome, Sjogren's syndrome).
Key features of the examination in adults include the following.
Infection: the important signs are fever, tachycardia, and hypotension. Characteristic signs of known infectious causes and sequelae should be sought.
Skin changes: many infections are associated with characteristic skin changes. Ecchymoses or petechiae due to thrombocytopenia are seen in haematological malignancies, aplastic anaemia, and myeloproliferative disorder. Chronic inflammatory diseases are associated with characteristic skin changes and joint deformities.
Lymphadenopathy: seen in haematological malignancies and infectious mononucleosis.
Splenomegaly: seen in haematological malignancies.
General history and examination in children
Children usually present with recurrent bacterial infections with or without failure to thrive. The most likely cause in children <2 years is primary autoimmune neutropenia in infancy. Neonatal alloimmune neutropenia should also be suspected in neonates. Infections and adverse drug reactions are also common causes, and become more important in older children. Acute lymphocytic leukaemia (ALL) can present in children <5 years. Congenital neutropenias are usually diagnosed in the first few months of life. Pseudoneutropenia should only be considered as a diagnosis in an asymptomatic patient.
Key features of the history in children include the following.
Family history: a history of immune neutropenia in a sibling or parent may be present. Most congenital abnormalities are inherited. A family history of leukaemia may be detected.
Infection: characteristic risk factors and symptoms of known infectious causes and sequelae should be sought.
Drug history: the use of any medication known to cause neutropenia should prompt suspicion of an adverse drug reaction.
Associated symptoms of anaemia (fatigue, dyspnoea, and dizziness) and thrombocytopenia (bleeding and easy bruising) should prompt consideration of ALL.
Key features of the examination in children include the following.
Evaluation of height and weight.
Infection: the important signs are fever, tachycardia, and hypotension. Characteristic signs of known infectious causes and sequelae should be sought.
Skin changes: many infections are associated with characteristic skin changes. Ecchymoses or petechiae due to thrombocytopenia are seen in ALL.
Lymphadenopathy: seen in ALL.
Splenomegaly: seen in ALL.
Hepatomegaly: seen in glycogen storage disease.
Neutropenia due to an infectious cause
A range of primary infections are known to cause neutropenia. The index of suspicion for a particular infection depends on the age of the patient. HIV, viral hepatitis, or obligate-intracellular bacterial infections are serious and must not be missed.
Respiratory syncytial virus infection, erythema infectiosum, rubella, rubeola, and varicella can be diagnosed clinically. Other infections present with non-specific symptoms and require confirmation by appropriate testing. It is important to be alert for malaria and leishmaniasis in patients with known risk factors for these infections.
[Figure caption and citation for the preceding image starts]: Important viral causes of neutropenia to exclude (IV, intravenous; RUQ, right upper quadrant)Created at BMJ Knowledge Centre [Citation ends].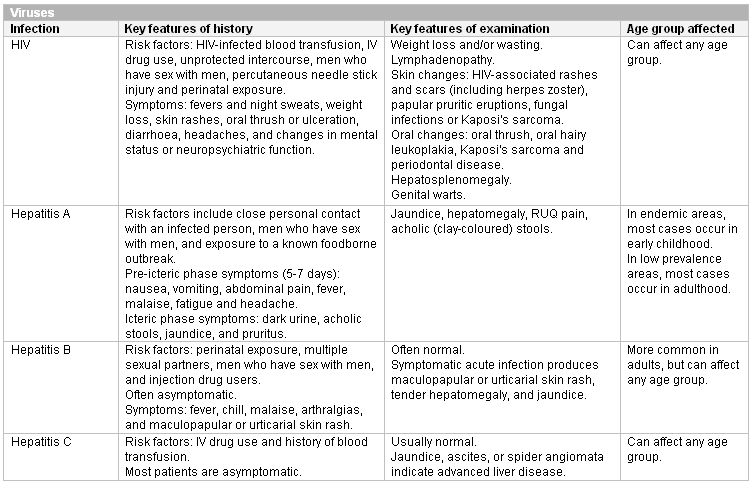 [Figure caption and citation for the preceding image starts]: Other viral causes of neutropenia in childhoodCreated at BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Other viral causes of neutropenia in childhoodCreated at BMJ Knowledge Centre [Citation ends].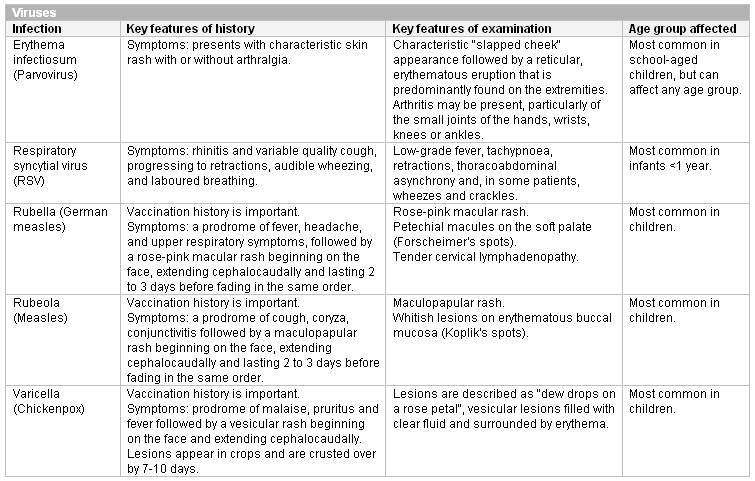 [Figure caption and citation for the preceding image starts]: Other viral causes of neutropenia at any ageCreated at BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Other viral causes of neutropenia at any ageCreated at BMJ Knowledge Centre [Citation ends].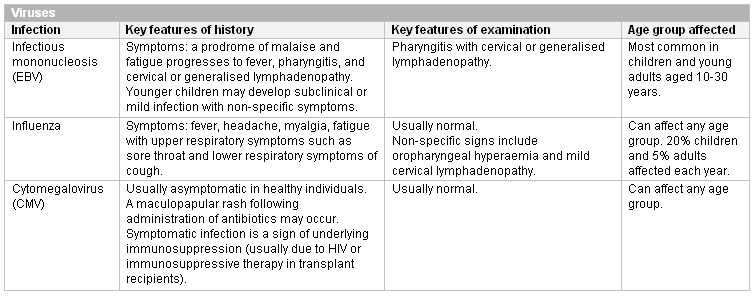 [Figure caption and citation for the preceding image starts]: Bacterial causes of neutropenia (bpm, beats per minute)Created at BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Bacterial causes of neutropenia (bpm, beats per minute)Created at BMJ Knowledge Centre [Citation ends].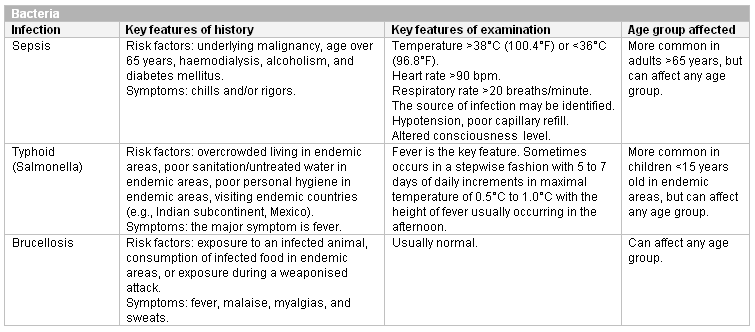 [Figure caption and citation for the preceding image starts]: Intracellular pathogens and parasites known to cause neutropeniaCreated at BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Intracellular pathogens and parasites known to cause neutropeniaCreated at BMJ Knowledge Centre [Citation ends].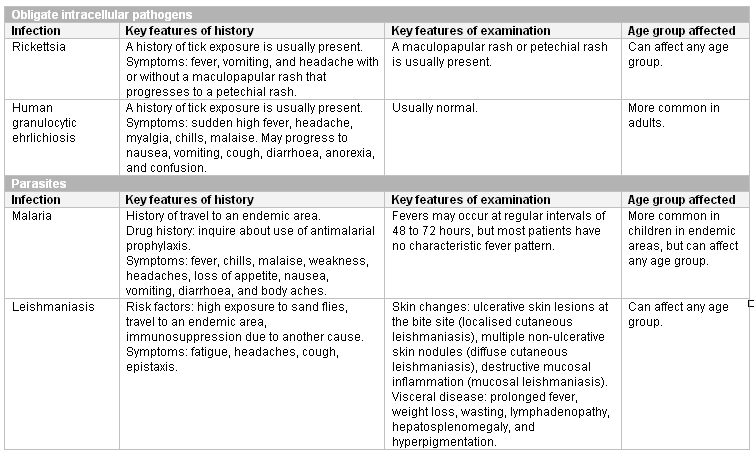
Secondary infection
Neutropenia increases the susceptibility to bacterial and fungal infection, which can be fatal. The most important general signs in these patients are fever, tachycardia, and hypotension, as the usual signs of inflammation may be absent or less obvious in neutropenic individuals.
The source of secondary infection is usually the endogenous flora of the gut and mucosa, commonly Staphylococcus and gram-negative organisms. Common sites of infection include mucous membranes (gingivitis, aphthous ulcers, stomatitis, perirectal abscesses), the skin (cellulitis), the liver (liver abscesses), the upper respiratory tract, and the lungs (pneumonia).[1][2] Oral candidiasis, and fungal skin and nail infections also occur. The risk of viral or parasitic infection is not increased, but asymptomatic infections such as cytomegalovirus can become symptomatic.
Sepsis
Sepsis is also an important cause of neutropenia. The most common site of infection is the lung, followed by infections of the bloodstream, abdomen, urinary tract, skin, and soft tissues. Rarer causes of sepsis that may need exclusion include meningitis and bacterial endocarditis.
Risk factors for sepsis include: age under 1 year, age over 75 years, frailty, impaired immunity (due to illness or drugs), recent surgery or other invasive procedures, any breach of skin integrity (e.g., cuts, burns), intravenous drug misuse, indwelling lines or catheters, and pregnancy or recent pregnancy.[47] See Sepsis in adults and Sepsis in children.
Drug-induced neutropenia
Drug-induced neutropenia is more common in patients over 60 years of age, and women are more commonly affected than men. The agents most likely to cause neutropenia are antithyroid medications, macrolides, and procainamides. Severe agranulocytosis from a drug reaction is rare and most frequently associated with clozapine, antithyroid drugs (thionamides), and sulfasalazine.[10][18][19][20][21] The neutropenia usually occurs within 1 to 2 days of starting the drug, and can appear within a few hours. It usually resolves in 1 to 3 weeks if the offending drug is discontinued. Late onset of neutropenia has been described after rituximab treatment.[17][Figure caption and citation for the preceding image starts]: Drugs and chemicals associated with neutropenia (ACE, angiotensin-converting enzyme)Created at BMJ Knowledge Centre based on author information [Citation ends].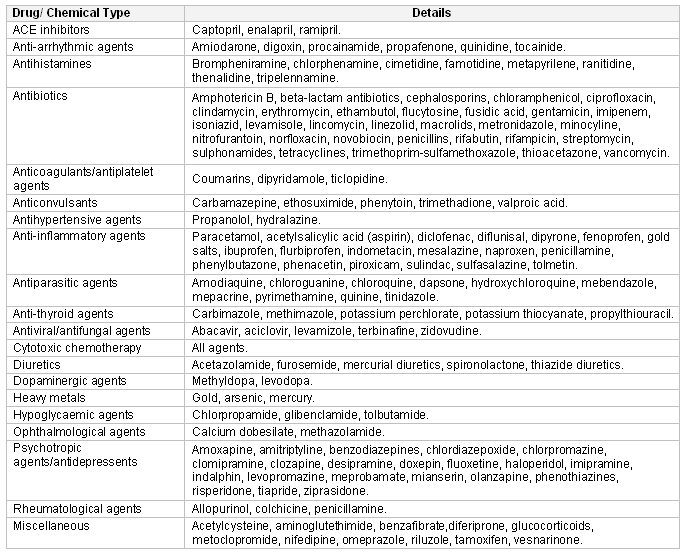
Congenital neutropenia
The most commonly encountered congenital diseases are severe congenital neutropenia and cyclic neutropenia.
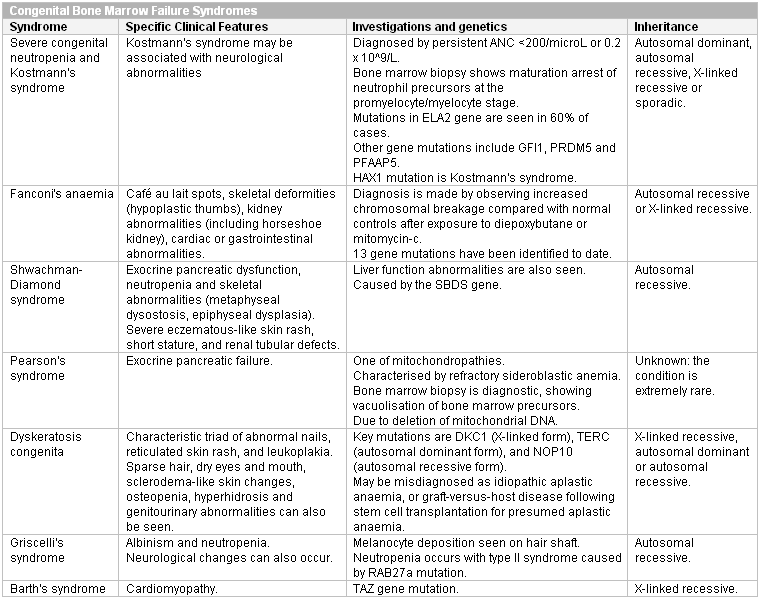
Severe congenital neutropenia: in most patients the diagnosis is suspected because of early onset of severe bacterial infections in the first year of life and an absolute neutrophil count (ANC) <200/microlitre or <0.2 x 10⁹/L.[34] The first symptom may be omphalitis (infection of the umbilical stump) occurring just after birth. Otitis media, pneumonitis, infections of the upper respiratory tract, and abscesses of the skin or liver are also common. Frequent aphthous stomatitis and gingival hyperplasia occur and can cause early loss of permanent teeth.
Cyclic neutropenia: characterised by a cycle of neutropenia that repeats every 21 days. Neutrophils are normal during the peaks. During the nadir, patients experience clinical manifestations of neutropenia, including malaise, fever, mucosal ulcers, cervical adenopathy, and abscesses of the axilla or groin.[2] A family history is common, but sporadic cases occur. Patients are usually healthy at birth but soon begin to have intermittent infections mostly of the respiratory tract. Compared with severe congenital neutropenia, patients are usually older at diagnosis and experience less severe infections. The diagnosis is usually made in childhood but can present in adults. The rare adult presentation is generally in association with clonal T-lymphocyte disorders, Crohn's disease, pregnancy, or exposure to radiation or certain drugs.[58]
Skin changes and dysmorphic features are seen in some of the rarer congenital neutropenias and may be apparent during the examination. Skin changes seen in the congenital neutropenias include cafe-au-lait spots (Fanconi anaemia), severe eczematous skin rashes (Shwachman-Diamond syndrome), and scleroderma-like changes (dyskeratosis congenita). Hypoplastic thumbs are seen in Fanconi anaemia. Short stature, metaphyseal dysostosis, and epiphyseal dysplasia are seen in Shwachman-Diamond syndrome. Nail changes are seen with dyskeratosis congenita.
Diagnostic tests in adults
General investigations and therapeutic trial
Neutropenia is confirmed by repeat testing with a FBC, differential, and manual examination of the peripheral blood smear. Lymphocytosis or monocytosis may be present in some conditions. Characteristic haematological abnormalities will be detected that can guide diagnosis.
In drug-induced neutropenia, discontinuation of the responsible drug will lead to resolution of the neutropenia in 1 to 3 weeks. No further testing is required, but other causes may need to be excluded in this time if there is a strong index of suspicion.
Suspected primary infection
Patients with suspected sepsis require thorough evaluation to determine the source of the infection, and to distinguish septic shock from other forms of shock. The key to early recognition is the systematic identification of any patient who has signs or symptoms suggestive of infection and is at risk of deterioration due to organ dysfunction. Several risk stratification approaches have been proposed. All rely on a structured clinical assessment and recording of the patient’s vital signs.[47][49][50][51][52] Check local guidance for information on which approach your institution recommends.
Typhoid (Salmonella) is diagnosed using blood cultures. Blood cultures should be obtained in all patients with features suspicious of infection.
Brucellosis is diagnosed using antibody serology and should be tested for if there are signs of infection.
Viral screen typically includes HIV, hepatitis A, B, and C, cytomegalovirus, and Epstein-Barr virus and should be performed in all patients. Parvovirus is also screened for if typical clinical features are absent.
Rickettsial infection and human granulocytic ehrlichiosis are diagnosed by antibody serology, but the antibodies only appear 7 to 10 days into the infection. These tests are only required if the diagnosis is suspected based on clinical features. If these conditions are suspected, treatment should be started presumptively.
If malaria is suspected based on risk factors and the clinical history, microscopy of erythrocytes is required and will detect intracellular asexual forms of the parasites. Good quality thick and thin films should be assessed.[59][60]
Cutaneous leishmaniasis is diagnosed by microscopy or polymerase chain reaction of skin scrapings. Visceral leishmaniasis is diagnosed using antibody serology. These tests are only required if risk factors and clinical findings suggest the diagnosis.
Suspected nutritional deficiency
Patients should have measurement of serum B12, folate, and copper levels.
B12 and folate deficiency will usually produce an associated anaemia.
If vitamin B12 and folate levels are normal in people with alcohol-use disorder, consider bone marrow biopsy to exclude alcohol-related bone marrow toxicity.
Suspected primary acquired bone marrow disease
These are serious diseases requiring prompt diagnosis and treatment.
FBC with differential and a blood smear will reveal a range of haematological abnormalities depending on the underlying condition and is required in all patients.
Erythrocyte sedimentation rate should be requested if lymphoma is suspected, as it is elevated in these patients and is related to prognosis.
Serum electrolytes are required in all patients. Hypercalcaemia and hyperphosphataemia may be present in haematological malignancies.
Renal and liver profiles are essential baseline investigations.
Serum uric acid and lactic dehydrogenase: may be elevated in haematological malignancy. Uric acid levels reflect the tumour burden.
Coagulation profile (prothrombin time, partial thromboplastin time, fibrinogen, D-dimers) is required if there are associated symptoms of excessive bleeding.
Flow cytometry: provides a definitive diagnosis of chronic lymphocytic leukaemia (CLL). This should be performed before bone marrow biopsy, as patients with CLL do not require bone marrow biopsy.
Lymph node biopsy: provides a definitive diagnosis of Hodgkin's and non-Hodgkin's lymphoma. Immunohistochemical studies can be added to distinguish these lymphomas.
Bone marrow aspiration: provides a definitive diagnosis of aplastic anaemia.
Bone marrow biopsy: for the definitive diagnosis of acute leukaemia (acute myelogenous leukaemia and ALL), myelodysplastic syndrome, aplastic anaemia, and bone marrow metastases. It is also used to stage lymphoma. Bone marrow biopsy is required in all patients in whom CLL has been excluded.
Additional phenotyping tests are used to confirm the diagnoses and to locate suitable donors for stem cell transplantation.
Suspected immune neutropenia
Antineutrophil antibodies are diagnostic. Repeated measurements are often required to obtain a positive result.
Chronic inflammatory disease
Antineutrophil antibodies may be present if these patients develop neutropenia. Repeated measurements are often required to obtain a positive result.
Bone marrow biopsy shows large granular lymphocyte infiltration, but is not required to assess neutropenia due to chronic inflammatory disease. If clinical features of primary acquired bone marrow disease are present, bone marrow biopsy can be performed to exclude these conditions.
Tests for the underlying condition are rarely needed as the diagnosis is usually established.
If required, the key diagnostic tests are rheumatoid factor or anti-complement control protein antibodies (rheumatoid arthritis and Felty's syndrome), antinuclear antibodies or anti-double-stranded DNA antibodies (SLE), and anti 60kDa Ro and La antibodies (Sjogren's syndrome) to diagnose the underlying diseases.
Suspected pseudoneutropenia
A manual differential is required to account for laboratory artifacts produced by increased agglutination or excessive delay prior to sample processing.
Pseudoneutropenia due to increased margination is a diagnosis of exclusion in an asymptomatic patient.
Diagnostic tests in children
The diagnostic strategy in children is different from that in adults. The most likely cause is an acquired immune neutropenia (either primary autoimmune neutropenia in infancy, or neonatal alloimmune neutropenia). If this is excluded, congenital neutropenias and haematological malignancy should be tested for. Clinical features suggestive of an infectious cause should prompt investigation of the infection. Any potential causative drugs should be discontinued.
Initial investigations include the following;.
FBC: as in adults, the neutropenia must be confirmed. The differential may reveal blasts (acute leukaemia) or specific appearances of neutrophils seen with congenital causes of neutropenia. Children with immune neutropenia may have a compensatory monocytosis accompanying severe neutropenia.
Antineutrophil antibodies: these are required to detect an immune neutropenia. This is especially likely if a previously healthy infant has a first episode of neutropenia at the age of 6 months or older, or if there is a family history of autoimmune neutropenia. If the test is positive, bone marrow biopsy is not required. If the test is negative, it must be repeated, as several measurements are often needed to obtain a positive result. The assay utilises free unbound antibody so the test will be negative if most of the antibody is adherent to the cell.
Absolute neutrophil count (ANC) is used to diagnose severe congenital neutropenia. Bone marrow biopsy is not required for diagnosis, but is required to monitor for malignant transformation.
Serial FBC measurements are used to diagnose cyclic neutropenia. Bone marrow biopsy is not required.
Bone marrow biopsy and genetic testing: if antineutrophil antibody testing is negative, and congenital neutropenia or cyclic neutropenia are not suspected, bone marrow biopsy and genetic testing should be considered to exclude ALL or the rarer congenital diseases. Congenital causes of neutropenia will usually present with recurrent or severe infections within the first year of life, and most present within the first 6 months. ALL tends to affect older children, with a peak in incidence at the age of 5 years.
Serum immunoglobulins: if antineutrophil testing is negative, and congenital neutropenia or cyclic neutropenia are not suspected, serum immunoglobulins should be measured to detect congenital immunodeficiency syndromes.
Diagnosis of infection: many childhood viruses can be diagnosed clinically (respiratory syncytial virus, rubella, rubeola, varicella). If symptoms are non-specific, the diagnostic approach is the same as for adults. HIV and hepatitis are important infections to exclude.
Results regarding the predictive value of serum biomarkers (including C-reactive protein, pro-calcitonin, and interleukin-6) in children and young people with cancer are inconsistent.[61] Current data do not support the use of these tests.
Identifying children with cancer with low-risk febrile neutropenia using a validated risk stratification strategy is recommended, however no clinical decision rule perfectly differentiates between children with febrile neutropenia at low or high risk of infection.[62]
Subsequent targeted diagnostic tests for congenital disease include the following.
Severe congenital neutropenia: the diagnosis is made by serial counts showing persistent ANC below 200/microlitre or 0.2 x 10⁹/L.[34] Antineutrophil antibodies should be tested to confirm that neutropenia is not immune-mediated. Other haematological abnormalities may include mild anaemia and thrombocytosis. Peripheral blood monocytosis and eosinophilia can occur. Bone marrow examination shows 'maturation arrest' of neutrophil precursors at the promyelocyte/myelocyte stage and may demonstrate increased numbers of eosinophils and monocytes. Cellularity is usually normal or slightly decreased and cytogenetics are usually normal.
Cyclic neutropenia: the diagnosis is made by repeated FBC measurement 3 times a week for 6 weeks. The FBC will reveal a characteristic cycling of the neutrophil count between the normal range and 0, repeating approximately every 21 days. Bone marrow biopsy is not required to make the diagnosis. If performed in the first few days of severe neutropenia, the marrow shows no development beyond the promyelocyte stage but will return to normal as the neutrophil count increases.[54]
Diagnosis of the rarer congenital causes often requires bone marrow biopsy and cytogenetics. The diagnosis of Fanconi anaemia is made by observing increased chromosomal breakage compared with normal controls after exposure to diepoxybutane or mitomycin-c. Dyskeratosis congenita is suspected clinically, but because the characteristic nail changes may not appear until the second decade of life, it may be misdiagnosed as acquired aplastic anaemia. If it is treated as acquired aplastic anaemia with allogeneic haematopoietic stem cell transplant, the characteristic nail changes may be misdiagnosed as graft-versus-host disease. The diagnosis is confirmed by cytogenetics. Griscelli's syndrome can be diagnosed by microscopic observation of melanocyte deposition on the hair shaft.
Chronic idiopathic neutropenia is a diagnosis of exclusion; it does not increase the risk of infection. Myelokathexis does not increase infection risk and is diagnosed from the characteristic morphology of neutrophils. Cohen's syndrome can be diagnosed clinically and confirmed by cytogenetics. Neutropenia occurring as part of this syndrome does not increase the risk of infection and requires no further investigation. Hermansky-Pudlak type 2 disease and P14 deficiency both produce albinism and are diagnosed using cytogenetics.
Immunodeficiency disorders should be screened for using an immunoglobulin screen. Some are suggested by characteristic clinical features. Confirmation of the diagnosis involves characteristic appearances of neutrophils, or cytogenetics.
Inborn errors of metabolism can be diagnosed using clinical picture screening algorithms such as those developed by the American College of Medical Genetics.[63][Figure caption and citation for the preceding image starts]: Congenital bone marrow failure syndromes (ANC, absolute neutrophil count)Created at BMJ Knowledge Centre [Citation ends].
 [Figure caption and citation for the preceding image starts]: Congenital isolated neutropeniasCreated at BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Congenital isolated neutropeniasCreated at BMJ Knowledge Centre [Citation ends].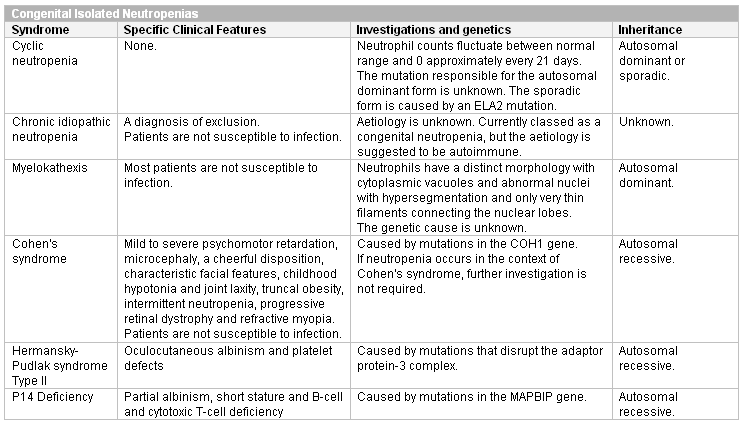 [Figure caption and citation for the preceding image starts]: Congenital immunodeficiency disorders (Ig, immunoglobulin)Created at BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Congenital immunodeficiency disorders (Ig, immunoglobulin)Created at BMJ Knowledge Centre [Citation ends].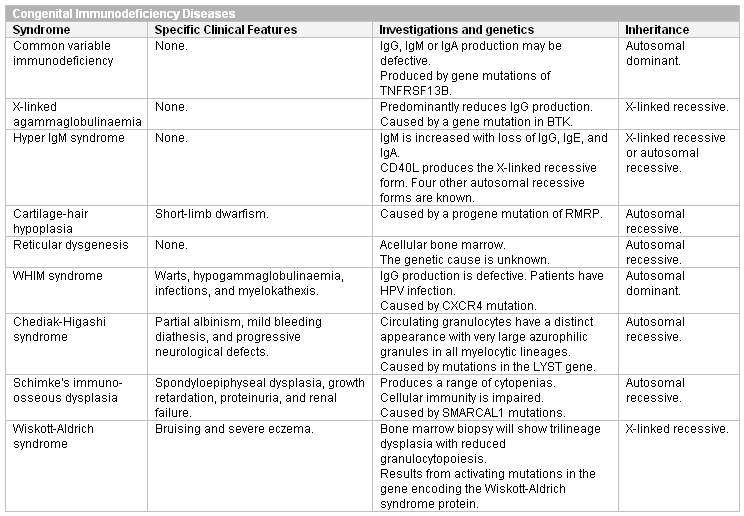
Use of this content is subject to our disclaimer