Acetaminophen overdose
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
Look out for this icon: for treatment options that are affected, or added, as a result of your patient's comorbidities.
acute single ingestion
acetylcysteine
Physicians are advised to consult the poison control center and/or medical toxicologist for guidance. Consider the need to assess and treat for co-ingestion of other drugs, particularly in cases of intentional overdose.
For a single acute ingestion of acetaminophen, patients should be transferred to the emergency department for evaluation and consideration of acetylcysteine.
If the estimated ingestion is unknown, >10 g, or ≥200 mg/kg (whichever is less), 2023 US and Canadian consensus guidelines recommend acetylcysteine should be started immediately, if waiting for testing will result in treatment starting more than 8 hours after ingestion.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com Other indications for administering acetylcysteine before the acetaminophen concentration is available include: if the patient is unconscious (and there is reasonable suspicion of acetaminophen toxicity), estimated ingestion is ≥30 g (high-risk ingestion), or if the patient presents with hepatotoxicity possibly from acetaminophen.
In practice, the initial dose is typically administered as soon as the need for treatment becomes evident.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com
The serum acetaminophen level should be checked 4 hours after ingestion.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com If a patient presents within 4 hours of ingestion, the level is drawn when 4 hours have elapsed since the possible overdose. Patients presenting between 4 and 8 hours after ingestion should have acetaminophen levels measured as soon as possible.
Once the acetaminophen level is obtained, it should be plotted against the time since ingestion on the revised Rumack-Matthew nomogram. [Figure caption and citation for the preceding image starts]: Revised Rumack-Matthew nomogram for the acute ingestion of acetaminophenDart RC et al. JAMA Netw Open. 2023;6(8):e2327739; used with permission [Citation ends].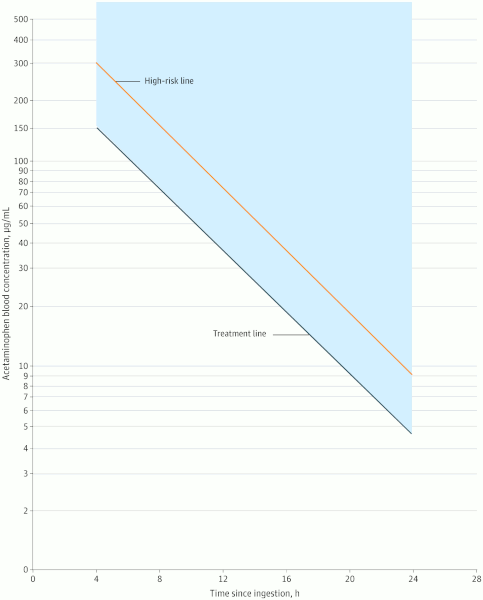
If the plot falls on or above the treatment line, the patient should be treated with acetylcysteine. It can be given either orally or intravenously.[76]Chiew AL, Gluud C, Brok J, et al. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev. 2018 Feb 23;(2):CD003328. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003328.pub3/full http://www.ncbi.nlm.nih.gov/pubmed/29473717?tool=bestpractice.com [89]Cattermole GN. Should N-acetylcysteine be administered orally or intravenously for the treatment of paracetamol overdose? Hong Kong J Emerg Med. 2009 Apr;16(2):106-16. https://journals.sagepub.com/doi/abs/10.1177/102490790901600209
If the plotted acetaminophen level falls below the treatment line on the Rumack-Matthew nomogram, then toxicity is exceedingly rare. Further evaluation and acetylcysteine is not required, or it can be discontinued.
Acetylcysteine is indicated beyond standard duration of therapy (21 hours for intravenous administration; 72 hours for oral administration) in patients with continued evidence of liver injury/dysfunction, abnormalities in predictors of poor prognosis, or persisting presence of serum acetaminophen detected by laboratory testing.[94]American College of Medical Toxicology. ACMT Position statement: duration of intravenous acetylcysteine therapy following acetaminophen overdose. J Med Toxicol. 2017 Mar;13(1):126-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5330952 http://www.ncbi.nlm.nih.gov/pubmed/26957510?tool=bestpractice.com Consult the poison control center for guidance on a suitable dose for these patients. End points for treatment with acetylcysteine in such cases are a clinically well-appearing patient with undetectable acetaminophen level <10 micrograms/mL, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) normal for the patient (or if elevated, have decreased from 25% to 50% from peak), and international normalized ratio (INR) <2.0.
Acetylcysteine dose regimens vary widely. If using an alternative regimen, it is recommended to use an approved or published intravenous or oral protocol that delivers a minimum of 300 mg/kg over 20-24 hours.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com Abbreviated courses of oral acetylcysteine should be used with caution only in early-presenting, well-appearing patients with favorable laboratory studies.
Rarely, hemodialysis is recommended in addition to treatment with acetylcysteine in a patient with a very large ingestion of acetaminophen (e.g., concentration of 900 micrograms/mL or greater with acidosis or altered consciousness due to acetaminophen toxic effects); consult with a poison center or toxicologist.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com The acetylcysteine infusion dose may need to be adjusted in patients on hemodialysis; consult your local drug information source for more information.
Primary options
acetylcysteine: children and adults: 150 mg/kg intravenous infusion over 1 hour, followed by 50 mg/kg infusion over 4 hours, then 100 mg/kg infusion over 16 hours; OR 140 mg/kg orally as a loading dose, followed by 70 mg/kg every 4 hours for 72 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
acetylcysteine: children and adults: 150 mg/kg intravenous infusion over 1 hour, followed by 50 mg/kg infusion over 4 hours, then 100 mg/kg infusion over 16 hours; OR 140 mg/kg orally as a loading dose, followed by 70 mg/kg every 4 hours for 72 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
acetylcysteine
supportive care
Treatment recommended for ALL patients in selected patient group
May include physiologic support such as intubation and ventilation or vasoactive infusions for blood pressure support, psychological care for mental illness, and education for those with accidental overdoses.
Supportive care must be tailored to each specific patient and is essential to good outcomes.
antiemetic
Treatment recommended for SOME patients in selected patient group
Vomiting that occurs during intravenous acetylcysteine administration will not affect the efficacy of treatment. It can be treated with an antiemetic (e.g., ondansetron).
If vomiting occurs within 1 hour of oral acetylcysteine, an antiemetic is administered and oral acetylcysteine dose is re-administered.
One randomized controlled trial found that while pretreatment with ondansetron was effective at decreasing vomiting, it had no effect on anaphylactoid reactions (also referred to as nonallergic anaphylactic reactions, or NAARs) and was associated with an increase in serum aminotransferase levels.[104]Bateman DN, Dear JW, Thanacoody HK, et al. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomised controlled trial. Lancet. 2014 Feb 22;383(9918):697-704. http://www.ncbi.nlm.nih.gov/pubmed/24290406?tool=bestpractice.com
Primary options
ondansetron: children: 0.1 mg/kg intravenously as a single dose, maximum 4 mg/dose; adults: 4-8 mg intravenously as a single dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
ondansetron: children: 0.1 mg/kg intravenously as a single dose, maximum 4 mg/dose; adults: 4-8 mg intravenously as a single dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
ondansetron
activated charcoal
Treatment recommended for SOME patients in selected patient group
Activated charcoal may be considered in a cooperative patient presenting within 4 hours of acute single acetaminophen ingestion; charcoal may decrease the need for acetylcysteine when given within 4 hours of ingestion.
Evidence (very low quality) suggests that activated charcoal is the best choice to reduce absorption of acetaminophen.[76]Chiew AL, Gluud C, Brok J, et al. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev. 2018 Feb 23;(2):CD003328. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003328.pub3/full http://www.ncbi.nlm.nih.gov/pubmed/29473717?tool=bestpractice.com Patients receiving activated charcoal at a median 2 hours post-ingestion of a large dose of acetaminophen (≥40 g) had significantly lower acetaminophen concentrations (measured ≥1 hour after charcoal) than those who did not.[78]Chiew AL, Isbister GK, Kirby KA, et al. Massive paracetamol overdose: an observational study of the effect of activated charcoal and increased acetylcysteine dose (ATOM-2). Clin Toxicol (Phila). 2017 Dec;55(10):1055-65. http://www.ncbi.nlm.nih.gov/pubmed/28644687?tool=bestpractice.com
Primary options
charcoal, activated: children: 0.5 to 1 g/kg orally/nasogastrically as a single dose, usual dose 10-50 g/dose (depending on age); adults: 25-100 g orally/nasogastrically as a single dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
charcoal, activated: children: 0.5 to 1 g/kg orally/nasogastrically as a single dose, usual dose 10-50 g/dose (depending on age); adults: 25-100 g orally/nasogastrically as a single dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
charcoal, activated
acetylcysteine
Physicians are advised to consult the poison control center and/or medical toxicologist for guidance. Consider the need to assess and treat for co-ingestion of other drugs, particularly in cases of intentional overdose.
Acetylcysteine should be started immediately if the patient presents >8 hours after ingestion. It can be given either intravenously or orally.[89]Cattermole GN. Should N-acetylcysteine be administered orally or intravenously for the treatment of paracetamol overdose? Hong Kong J Emerg Med. 2009 Apr;16(2):106-16. https://journals.sagepub.com/doi/abs/10.1177/102490790901600209 Obtain an acetaminophen level as soon as possible, as well as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and international normalized ratio (INR).
For patients with acetaminophen levels that plot on or above the treatment line on the revised Rumack-Matthew nomogram, the patient should have acetylcysteine continued.
If the plotted acetaminophen level falls below the treatment line on the Rumack-Matthew nomogram, the patient is asymptomatic, and there is no evidence of renal injury or liver damage/dysfunction (normal laboratory tests), acetylcysteine may be discontinued.
Acetylcysteine is indicated beyond standard duration of therapy (21 hours for intravenous administration; 72 hours for oral administration) in patients with continued evidence of liver injury/dysfunction, abnormalities in predictors of poor prognosis, or persisting presence of serum acetaminophen detected by laboratory testing.[94]American College of Medical Toxicology. ACMT Position statement: duration of intravenous acetylcysteine therapy following acetaminophen overdose. J Med Toxicol. 2017 Mar;13(1):126-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5330952 http://www.ncbi.nlm.nih.gov/pubmed/26957510?tool=bestpractice.com Consult the poison control center for guidance on a suitable dose for these patients. End points for treatment with acetylcysteine in such cases are a well-appearing patient, acetaminophen level <10 micrograms/mL, serum AST or ALT (normal for the patient or, if elevated, have decreased from peak by 25% to 50%), and INR <2.0.
Acetylcysteine dose regimens vary widely. If using an alternative regimen, it is recommended to use an approved or published intravenous or oral protocol that delivers a minimum of 300 mg/kg over 20-24 hours.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com
Rarely, hemodialysis is recommended in addition to treatment with acetylcysteine for patients with a very large ingestion of acetaminophen (e.g., concentration of 900 micrograms/mL or greater with acidosis or altered consciousness due to acetaminophen toxic effects); consult with a poison center or toxicologist.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com The acetylcysteine infusion dose may need to be adjusted in patients on hemodialysis; consult your local drug information source for more information.
Primary options
acetylcysteine: children and adults: 150 mg/kg intravenous infusion over 1 hour, followed by 50 mg/kg infusion over 4 hours, then 100 mg/kg infusion over 16 hours; OR 140 mg/kg orally as a loading dose, followed by 70 mg/kg every 4 hours for 72 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
acetylcysteine: children and adults: 150 mg/kg intravenous infusion over 1 hour, followed by 50 mg/kg infusion over 4 hours, then 100 mg/kg infusion over 16 hours; OR 140 mg/kg orally as a loading dose, followed by 70 mg/kg every 4 hours for 72 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
acetylcysteine
supportive care
Treatment recommended for ALL patients in selected patient group
May include physiologic support such as intubation and ventilation or vasoactive infusions for blood pressure support, psychological care for mental illness, and education for those with accidental overdoses.
Supportive care must be tailored to each specific patient and is essential to good outcomes.
antiemetic
Treatment recommended for SOME patients in selected patient group
Vomiting that occurs during intravenous acetylcysteine administration will not affect the efficacy of treatment. It can be treated with an antiemetic (e.g., ondansetron).
If vomiting occurs within 1 hour of oral acetylcysteine, an antiemetic is administered and oral acetylcysteine dose is re-administered.
One randomized controlled trial found that while pretreatment with ondansetron was effective at decreasing vomiting, it had no effect on anaphylactoid reactions (also referred to as nonallergic anaphylactic reactions, or NAARs) and was associated with an increase in serum aminotransferase levels.[104]Bateman DN, Dear JW, Thanacoody HK, et al. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomised controlled trial. Lancet. 2014 Feb 22;383(9918):697-704. http://www.ncbi.nlm.nih.gov/pubmed/24290406?tool=bestpractice.com
Primary options
ondansetron: children: 0.1 mg/kg intravenously as a single dose, maximum 4 mg/dose; adults: 4-8 mg intravenously as a single dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
ondansetron: children: 0.1 mg/kg intravenously as a single dose, maximum 4 mg/dose; adults: 4-8 mg intravenously as a single dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
ondansetron
evaluation for liver transplant
Treatment recommended for SOME patients in selected patient group
Laboratory criteria are helpful when evaluating patients for liver transplantation due to acetaminophen hepatotoxicity. However, patients with significant hepatotoxicity should be referred to a liver transplant facility as early as possible, ideally before metabolic acidosis, coagulopathy, and encephalopathy occur.
Referral for liver transplant is indicated if arterial pH <7.3 AND lactate >3.0 mmol/L after fluid resuscitation, OR prothrombin time (PT)/international normalized ratio (INR) >100 seconds/6.0 seconds AND encephalopathy grade 3 or more AND creatinine >3.3 mg/dL (292 micromol/L) within 24 hours AND a normal arterial pH.[58]O'Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989 Aug;97(2):439-45. http://www.ncbi.nlm.nih.gov/pubmed/2490426?tool=bestpractice.com [99]Bernal W, Donaldson N, Wyncoll D, et al. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002 Feb 16;359(9306):558-63. http://www.ncbi.nlm.nih.gov/pubmed/11867109?tool=bestpractice.com
Other predictors of poor prognosis without transplant include arterial lactate concentration >3.5 mmol/L after fluid resuscitation (<4 hours) OR lactate >3 mmol/L after full fluid resuscitation OR phosphate >3.75 mg/dL (>1.2 mmol/L) at 48-96 hours.[58]O'Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989 Aug;97(2):439-45. http://www.ncbi.nlm.nih.gov/pubmed/2490426?tool=bestpractice.com [99]Bernal W, Donaldson N, Wyncoll D, et al. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002 Feb 16;359(9306):558-63. http://www.ncbi.nlm.nih.gov/pubmed/11867109?tool=bestpractice.com
acetylcysteine
Physicians are advised to consult the poison control center and/or medical toxicologist for guidance. Consider the need to assess and treat for co-ingestion of other drugs, particularly in cases of intentional overdose.
Acetylcysteine should be started immediately if the patient presents >24 hours after ingestion. It can be given either intravenously or orally.[89]Cattermole GN. Should N-acetylcysteine be administered orally or intravenously for the treatment of paracetamol overdose? Hong Kong J Emerg Med. 2009 Apr;16(2):106-16. https://journals.sagepub.com/doi/abs/10.1177/102490790901600209 Obtain an acetaminophen level as soon as possible, as well as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and international normalized ratio (INR).
For patients with acetaminophen >10 micrograms/mL or abnormal AST/ALT (unless baseline for patient), acetylcysteine is continued. If acetaminophen is <10 micrograms/mL and AST/ALT normal (or baseline for patient), and the patient is clinically well-appearing, acetylcysteine may be discontinued.
Acetylcysteine is indicated beyond standard duration of therapy (21 hours for intravenous administration; 72 hours for oral administration) in patients with continued evidence of liver injury/dysfunction, abnormalities in predictors of poor prognosis, or persisting presence of serum acetaminophen detected by laboratory testing.[94]American College of Medical Toxicology. ACMT Position statement: duration of intravenous acetylcysteine therapy following acetaminophen overdose. J Med Toxicol. 2017 Mar;13(1):126-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5330952 http://www.ncbi.nlm.nih.gov/pubmed/26957510?tool=bestpractice.com Consult the poison control center for guidance on a suitable dose for these patients. End points for treatment with acetylcysteine in such cases are a clinically well-appearing patient with undetectable acetaminophen level <10 micrograms/mL, AST and ALT normal for the patient (or if elevated, have decreased from 25% to 50% from peak), and INR <2.
Acetylcysteine dose regimens vary widely. If using an alternative regimen, it is recommended to use an approved or published intravenous or oral protocol that delivers a minimum of 300 mg/kg over 20-24 hours.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com
Rarely, hemodialysis is recommended in addition to treatment with acetylcysteine for patients with a very large ingestion of acetaminophen (e.g., concentration of 900 micrograms/mL or greater with acidosis or altered consciousness due to acetaminophen toxic effects); consult with a poison center or toxicologist.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com The acetylcysteine infusion dose may need to be adjusted in patients on hemodialysis; consult your local drug information source for more information.
Primary options
acetylcysteine: children and adults: 150 mg/kg intravenous infusion over 1 hour, followed by 50 mg/kg infusion over 4 hours, then 100 mg/kg infusion over 16 hours; OR 140 mg/kg orally as a loading dose, followed by 70 mg/kg every 4 hours for 72 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
acetylcysteine: children and adults: 150 mg/kg intravenous infusion over 1 hour, followed by 50 mg/kg infusion over 4 hours, then 100 mg/kg infusion over 16 hours; OR 140 mg/kg orally as a loading dose, followed by 70 mg/kg every 4 hours for 72 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
acetylcysteine
supportive care
Treatment recommended for ALL patients in selected patient group
May include physiologic support such as intubation and ventilation or vasoactive infusions for blood pressure support, management of coagulopathy, and renal support for hepatorenal syndrome if present; psychological care for mental illness; and education for those with accidental overdoses.
Supportive care must be tailored to each specific patient and is essential to good outcomes.
antiemetic
Treatment recommended for SOME patients in selected patient group
Vomiting that occurs during intravenous acetylcysteine administration will not affect the efficacy of treatment. It can be treated with an antiemetic (e.g., ondansetron).
If vomiting occurs within 1 hour of oral acetylcysteine, an antiemetic is administered and oral acetylcysteine dose is re-administered.
One randomized controlled trial found that while pretreatment with ondansetron was effective at decreasing vomiting, it had no effect on anaphylactoid reactions (also referred to as nonallergic anaphylactic reactions, or NAARs) and was associated with an increase in serum aminotransferase levels.[104]Bateman DN, Dear JW, Thanacoody HK, et al. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomised controlled trial. Lancet. 2014 Feb 22;383(9918):697-704. http://www.ncbi.nlm.nih.gov/pubmed/24290406?tool=bestpractice.com
Primary options
ondansetron: children: 0.1 mg/kg intravenously as a single dose, maximum 4 mg/dose; adults: 4-8 mg intravenously as a single dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
ondansetron: children: 0.1 mg/kg intravenously as a single dose, maximum 4 mg/dose; adults: 4-8 mg intravenously as a single dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
ondansetron
evaluation for liver transplant
Treatment recommended for SOME patients in selected patient group
Laboratory criteria are helpful when evaluating patients for liver transplantation due to acetaminophen hepatotoxicity. However, patients with significant hepatotoxicity should be referred to a liver transplant facility as early as possible, ideally before metabolic acidosis, coagulopathy, and encephalopathy occur.
Referral for liver transplant is indicated if arterial pH <7.3 AND lactate >3.0 mmol/L after fluid resuscitation, OR prothrombin time (PT)/international normalized ratio (INR) >100 seconds/6.0 seconds AND encephalopathy grade 3 or more AND creatinine >3.3 mg/dL (292 micromol/L) within 24 hours AND a normal arterial pH.[58]O'Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989 Aug;97(2):439-45. http://www.ncbi.nlm.nih.gov/pubmed/2490426?tool=bestpractice.com [99]Bernal W, Donaldson N, Wyncoll D, et al. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002 Feb 16;359(9306):558-63. http://www.ncbi.nlm.nih.gov/pubmed/11867109?tool=bestpractice.com
Other predictors of poor prognosis without transplant include arterial lactate concentration >3.5 mmol/L after fluid resuscitation (<4 hours) OR lactate >3 mmol/L after full fluid resuscitation OR phosphate >3.75 mg/dL (>1.2 mmol/L) at 48-96 hours.[58]O'Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989 Aug;97(2):439-45. http://www.ncbi.nlm.nih.gov/pubmed/2490426?tool=bestpractice.com [99]Bernal W, Donaldson N, Wyncoll D, et al. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002 Feb 16;359(9306):558-63. http://www.ncbi.nlm.nih.gov/pubmed/11867109?tool=bestpractice.com
repeated supratherapeutic acetaminophen ingestion
acetylcysteine
Physicians are advised to consult the poison control center and/or medical toxicologist for guidance.
Repeated supratherapeutic ingestion is defined as multiple episodes of acetaminophen ingestion over a period greater than 24 hours that results any of the following: findings consistent with acetaminophen toxicity (repeated vomiting, right upper quadrant abdominal tenderness, or mental status changes); >6 g/day or >150 mg/kg/day (whichever is less) over period of 24-48 hours; >4 g/day or >100 mg/kg/day (whichever is less) over >48 hours.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com Discussion with a medical toxicologist or poison control center is strongly advised.
Acetaminophen levels should be measured as soon as possible.
For patients with signs or symptoms of hepatotoxicity, acetylcysteine should be started immediately, while awaiting laboratory results and any other necessary evaluation to exclude other differential diagnoses. It can be given either intravenously or orally.[89]Cattermole GN. Should N-acetylcysteine be administered orally or intravenously for the treatment of paracetamol overdose? Hong Kong J Emerg Med. 2009 Apr;16(2):106-16. https://journals.sagepub.com/doi/abs/10.1177/102490790901600209
For all other patients, acetylcysteine should be started if acetaminophen level is ≥20 micrograms/mL, or aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level is abnormal (unless abnormal aminotransferase level is baseline for the patient).[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com It can be given either orally or intravenously.[89]Cattermole GN. Should N-acetylcysteine be administered orally or intravenously for the treatment of paracetamol overdose? Hong Kong J Emerg Med. 2009 Apr;16(2):106-16. https://journals.sagepub.com/doi/abs/10.1177/102490790901600209
For patients with detectable acetaminophen levels, or any indication of acute kidney injury or liver injury/dysfunction, acetylcysteine is continued. If there is no detectable acetaminophen, and no evidence of renal injury or liver damage/dysfunction (normal laboratory tests), and the patient is asymptomatic, acetylcysteine may be discontinued.
Acetylcysteine is indicated beyond standard duration of therapy (21 hours for intravenous administration; 72 hours for oral administration) in patients with continued evidence of liver injury/dysfunction, abnormalities in predictors of poor prognosis, or persisting presence of serum acetaminophen detected by laboratory testing.[94]American College of Medical Toxicology. ACMT Position statement: duration of intravenous acetylcysteine therapy following acetaminophen overdose. J Med Toxicol. 2017 Mar;13(1):126-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5330952 http://www.ncbi.nlm.nih.gov/pubmed/26957510?tool=bestpractice.com Consult the poison control center for guidance on a suitable dose for these patients. End points for treatment with acetylcysteine in such cases are asymptomatic clinical status, undetectable acetaminophen level, serum AST or ALT (normal for the patient or, if elevated, have decreased from peak by 25% to 50%), and international normalized ratio (INR) <2.0.
Acetylcysteine dose regimens vary widely. If using an alternative regimen, it is recommended to use an approved or published intravenous or oral protocol that delivers a minimum of 300 mg/kg over 20-24 hours.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com
Rarely, hemodialysis is recommended in addition to treatment with acetylcysteine for patients with a very large ingestion of acetaminophen (e.g., concentration of 900 micrograms/mL or greater with acidosis or altered consciousness due to acetaminophen toxic effects); consult with a poison center or toxicologist.[1]Dart RC, Mullins ME, Matoushek T, et al. Management of acetaminophen poisoning in the US and Canada: a consensus statement. JAMA Netw Open. 2023 Aug 1;6(8):e2327739. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2808062 http://www.ncbi.nlm.nih.gov/pubmed/37552484?tool=bestpractice.com The acetylcysteine infusion dose may need to be adjusted in patients on hemodialysis; consult your local drug information source for more information.
Primary options
acetylcysteine: children and adults: 150 mg/kg intravenous infusion over 1 hour, followed by 50 mg/kg infusion over 4 hours, then 100 mg/kg infusion over 16 hours; OR 140 mg/kg orally as a loading dose, followed by 70 mg/kg every 4 hours for 72 hours
These drug options and doses relate to a patient with no comorbidities.
Primary options
acetylcysteine: children and adults: 150 mg/kg intravenous infusion over 1 hour, followed by 50 mg/kg infusion over 4 hours, then 100 mg/kg infusion over 16 hours; OR 140 mg/kg orally as a loading dose, followed by 70 mg/kg every 4 hours for 72 hours
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
acetylcysteine
supportive care
Treatment recommended for ALL patients in selected patient group
May include physiologic support such as intubation and ventilation or vasoactive infusions for blood pressure support, management of coagulopathy, and renal support for hepatorenal syndrome; psychological care for mental illness; and education for those with accidental overdoses.
Supportive care must be tailored to each specific patient and is essential to good outcomes.
antiemetic
Treatment recommended for SOME patients in selected patient group
Vomiting that occurs during intravenous acetylcysteine administration will not affect the efficacy of treatment. It can be treated with an antiemetic (e.g., ondansetron).
If vomiting occurs within 1 hour of oral acetylcysteine, an antiemetic is administered and oral acetylcysteine dose is re-administered.
One randomized controlled trial found that while pretreatment with ondansetron was effective at decreasing vomiting, it had no effect on anaphylactoid reactions (also referred to as nonallergic anaphylactic reactions, or NAARs) and was associated with an increase in serum aminotransferase levels.[104]Bateman DN, Dear JW, Thanacoody HK, et al. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomised controlled trial. Lancet. 2014 Feb 22;383(9918):697-704. http://www.ncbi.nlm.nih.gov/pubmed/24290406?tool=bestpractice.com
Primary options
ondansetron: children: 0.1 mg/kg intravenously as a single dose, maximum 4 mg/dose; adults: 4-8 mg intravenously as a single dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
ondansetron: children: 0.1 mg/kg intravenously as a single dose, maximum 4 mg/dose; adults: 4-8 mg intravenously as a single dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
ondansetron
evaluation for liver transplant
Treatment recommended for SOME patients in selected patient group
Laboratory criteria are helpful when evaluating patients for liver transplantation due to acetaminophen hepatotoxicity. However, patients with significant hepatotoxicity should be referred to a liver transplant facility as early as possible, ideally before metabolic acidosis, coagulopathy, and encephalopathy occur.
Referral for liver transplant is indicated if arterial pH <7.3 AND lactate >3.0 mmol/L after fluid resuscitation, OR prothrombin time (PT)/international normalized ratio (INR) >100 seconds/6.0 seconds AND encephalopathy grade 3 or more AND creatinine >3.3 mg/dL (292 micromol/L) within 24 hours AND a normal arterial pH.[58]O'Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989 Aug;97(2):439-45. http://www.ncbi.nlm.nih.gov/pubmed/2490426?tool=bestpractice.com [99]Bernal W, Donaldson N, Wyncoll D, et al. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002 Feb 16;359(9306):558-63. http://www.ncbi.nlm.nih.gov/pubmed/11867109?tool=bestpractice.com
Other predictors of poor prognosis without transplant include arterial lactate concentration >3.5 mmol/L after fluid resuscitation (<4 hours) OR lactate >3 mmol/L after full fluid resuscitation, OR phosphate >3.75 mg/dL (>1.2 mmol/L) at 48-96 hours.[58]O'Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989 Aug;97(2):439-45. http://www.ncbi.nlm.nih.gov/pubmed/2490426?tool=bestpractice.com [99]Bernal W, Donaldson N, Wyncoll D, et al. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002 Feb 16;359(9306):558-63. http://www.ncbi.nlm.nih.gov/pubmed/11867109?tool=bestpractice.com

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer
