If present, varices are usually detected on screening endoscopy as a part of the workup for patients with cirrhosis.
In patients without hemorrhage, varices may be asymptomatic. In these patients an evaluation for signs and symptoms of liver disease and extent of cirrhosis will help stratify risk for bleeding. Several predictive rules for early detection of esophageal varices have been proposed in the last few years, based mainly on liver and spleen stiffness, spleen diameter, platelet count, and hepatic venous pressure gradient (HVPG).[5]Kaplan DE, Ripoll C, Thiele M, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024 May 1;79(5):1180-211.
https://journals.lww.com/hep/fulltext/2024/05000/aasld_practice_guidance_on_risk_stratification_and.22.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37870298?tool=bestpractice.com
[29]Berzigotti A, Seijo S, Arena U, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013 Jan;144(1):102-11.e1.
http://www.ncbi.nlm.nih.gov/pubmed/23058320?tool=bestpractice.com
[30]Colecchia A, Montrone L, Scaioli E, et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012 Sep;143(3):646-54.
http://www.ncbi.nlm.nih.gov/pubmed/22643348?tool=bestpractice.com
[31]Takuma Y, Nouso K, Morimoto Y, et al. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013 Jan;144(1):92-101.e2.
http://www.ncbi.nlm.nih.gov/pubmed/23022955?tool=bestpractice.com
HVPG is now the gold standard method to assess the presence of clinically significant portal hypertension, defined as HVPG ≥10 mmHg.[5]Kaplan DE, Ripoll C, Thiele M, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024 May 1;79(5):1180-211.
https://journals.lww.com/hep/fulltext/2024/05000/aasld_practice_guidance_on_risk_stratification_and.22.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37870298?tool=bestpractice.com
However, noninvasive or minimally invasive techniques to assess portal hypertension have been proposed and are well established.[32]Ravaioli F, Montagnani M, Lisotti A, et al. Noninvasive assessment of portal hypertension in advanced chronic liver disease: an update. Gastroenterol Res Pract. 2018 Jun 7;2018:4202091.
https://www.hindawi.com/journals/grp/2018/4202091
http://www.ncbi.nlm.nih.gov/pubmed/29977287?tool=bestpractice.com
Active bleeding typically presents with hematemesis and/or melena. For patients presenting with acute upper gastrointestinal bleeding (UGIB), other causes should be considered in the history (e.g., peptic ulceration, gastric erosions, and adverse drug effects). Because patients with UGIB can experience rapid clinical deterioration, blood should be sent for typing and cross-matching in the event that blood products become necessary. Prompt diagnosis (and intervention) is essential to improve the clinical outcome.
Medical history
If possible, a full history should be taken to establish the cause of liver disease. Particular enquiries regarding alcohol abuse and possible viral hepatitis should be made. HIV infection may accelerate liver disease in patients with chronic viral hepatitis.
Autoimmune liver disease, hemochromatosis, Wilson disease, and other recognized causes of portal hypertension, such as Budd-Chiari syndrome, myeloproliferative disorders, and sarcoidosis, may contribute to esophageal variceal risk.
Clinical assessment
Physical examination may show signs of chronic liver disease such as ascites, jaundice, and splenomegaly. Other signs include spider angioma, bruising, petechiae, caput medusa, and tremor flapping. There may be evidence of encephalopathy.
Active variceal bleeding typically presents with hematemesis and/or melena.
Laboratory investigations
Blood should be sent for complete blood count, metabolic panel, liver function tests, and coagulation profile. Hepatitis B surface antigen and anti-hepatitis C virus IgG should be performed if the history suggests viral hepatitis as a cause of cirrhosis. Blood should be sent for typing and cross-matching.
Decompensated cirrhosis (Child-Pugh class B/C) predicts development and progression of esophageal varices, and also hemorrhage.[5]Kaplan DE, Ripoll C, Thiele M, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024 May 1;79(5):1180-211.
https://journals.lww.com/hep/fulltext/2024/05000/aasld_practice_guidance_on_risk_stratification_and.22.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37870298?tool=bestpractice.com
[9]Merli M, Nicolini G, Angeloni S, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003 Mar;38(3):266-72.
http://www.ncbi.nlm.nih.gov/pubmed/12586291?tool=bestpractice.com
Child-Pugh score should be calculated based on biochemical and clinical findings, as described below, and the Child-Pugh class determined.
[
Child Pugh classification for severity of liver disease
Opens in new window
]
Child-Pugh scoring uses five clinical measures of liver disease. Each measure is scored as between 1 and 3 points, with 3 indicating the most severe derangement. The clinical measures are:
Encephalopathy
None: 1 point
Grade 1 to 2: 2 points
Grade 3 to 4: 3 points
Ascites
None: 1 point
Mild/moderate: 2 points
Tense: 3 points
Bilirubin (mg/dL)
<2: 1 point
2 to 3: 2 points
>3: 3 points
Albumin (g/dL)
>3.5: 1 point
2.8 to 3.5: 2 points
<2.8: 3 points
International normalized ratio
<1.7: 1 point
1.7 to 2.3: 2 points
>2.3: 3 points.
Chronic liver disease is classified into Child-Pugh class A to C using the scores as above:
Imaging
Esophago-gastro-duodenoscopy (EGD) is considered the most accurate method to identify varices.[5]Kaplan DE, Ripoll C, Thiele M, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024 May 1;79(5):1180-211.
https://journals.lww.com/hep/fulltext/2024/05000/aasld_practice_guidance_on_risk_stratification_and.22.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37870298?tool=bestpractice.com
[6]The Italian Liver Cirrhosis Project. Reliability of endoscopy in the assessment of variceal features. J Hepatol. 1987 Feb;4(1):93-8.
http://www.ncbi.nlm.nih.gov/pubmed/3494762?tool=bestpractice.com
[33]Calès P, Zabotto B, Meskens C, et al. Gastroesophageal endoscopic features in cirrhosis. Observer variability, interassociations, and relationship to hepatic dysfunction. Gastroenterology. 1990 Jan;98(1):156-62.
http://www.ncbi.nlm.nih.gov/pubmed/2293575?tool=bestpractice.com
[34]Berzigotti A, Bosch J, Boyer TD. Use of noninvasive markers of portal hypertension and timing of screening endoscopy for gastroesophageal varices in patients with chronic liver disease. Hepatology. 2014 Feb;59(2):729-31.
https://aasldpubs.onlinelibrary.wiley.com/doi/full/10.1002/hep.26652
http://www.ncbi.nlm.nih.gov/pubmed/23913844?tool=bestpractice.com
Variceal size is the most important predictor of hemorrhage, with the highest risk of first hemorrhage occurring in patients with large varices (15% per year).[3]North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988 Oct 13;319(15):983-9.
http://www.ncbi.nlm.nih.gov/pubmed/3262200?tool=bestpractice.com
[4]Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010 Mar 4;362(9):823-32.
http://www.ncbi.nlm.nih.gov/pubmed/20200386?tool=bestpractice.com
The endoscopic finding of red wale marks (defined as longitudinal dilated venules resembling whip marks on the variceal surface) is also an important predictor.[3]North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988 Oct 13;319(15):983-9.
http://www.ncbi.nlm.nih.gov/pubmed/3262200?tool=bestpractice.com
[5]Kaplan DE, Ripoll C, Thiele M, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024 May 1;79(5):1180-211.
https://journals.lww.com/hep/fulltext/2024/05000/aasld_practice_guidance_on_risk_stratification_and.22.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37870298?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: (A) Grade I esophageal varices. These collapse to inflation of the esophagus with air. (B) Grade II esophageal varices. These are varices between grades 1 and 3. (C) Grade III esophageal varices. These are large enough to occlude the lumenTripathi D et al. Gut 2015;64:1680-704; used with permission [Citation ends].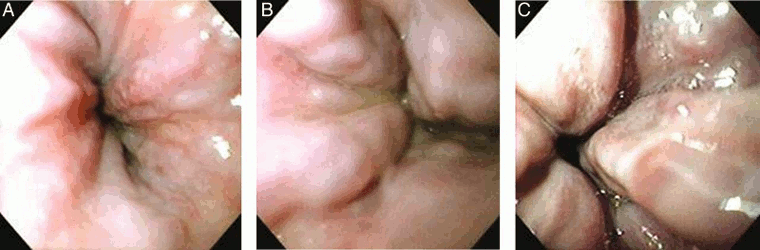
Identifying patients with a low probability of having high-risk gastroesophageal varices can help to avoid screening EGD (endoscopy). Expanded-Baveno VI criteria have been validated in several patient cohorts (with compensated advanced chronic liver disease) and suggest that endoscopy may only be indicated if liver stiffness measurement (LSM) ≥25 kPa and platelet count ≤110 ×10⁹ cells/L.[35]Augustin S, Pons M, Maurice JB, et al. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017 Dec;66(6):1980-8.
https://aasldpubs.onlinelibrary.wiley.com/doi/full/10.1002/hep.29363
http://www.ncbi.nlm.nih.gov/pubmed/28696510?tool=bestpractice.com
This prediction rule would potentially avoid 40% of endoscopies, with an associated risk of missing 0.6% (95% CI 0.3% to 1.4%) of varices requiring treatment among patients with compensated advanced chronic liver disease.[35]Augustin S, Pons M, Maurice JB, et al. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017 Dec;66(6):1980-8.
https://aasldpubs.onlinelibrary.wiley.com/doi/full/10.1002/hep.29363
http://www.ncbi.nlm.nih.gov/pubmed/28696510?tool=bestpractice.com
LSM by transient elastography is a noninvasive test carried out by applying a vibrating probe to the thoracic wall at the level of the right liver lobe.[36]Del Poggio P, Colombo S. Is transient elastography a useful tool for screening liver disease? World J Gastroenterol. 2009 Mar 28;15(12):1409-14.
https://pmc.ncbi.nlm.nih.gov/articles/PMC2665133
http://www.ncbi.nlm.nih.gov/pubmed/19322911?tool=bestpractice.com
Patients with hematemesis, hemodynamically unstable patients at risk of having blood in the stomach, or patients presenting with an altered mental status (i.e., confusion) should be intubated before endoscopy.[37]de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII - renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
https://www.journal-of-hepatology.eu/article/S0168-8278(21)02299-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35120736?tool=bestpractice.com
[38]Tripathi D, Stanley AJ, Hayes PC, et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015 Nov;64(11):1680-704.
https://gut.bmj.com/content/64/11/1680.long
http://www.ncbi.nlm.nih.gov/pubmed/25887380?tool=bestpractice.com
Patients with an LSM <20 kPa and platelet count >150,000/mm³ have a very low probability (<5%) of having high‐risk varices; therefore EGD can be safely avoided.[5]Kaplan DE, Ripoll C, Thiele M, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024 May 1;79(5):1180-211.
https://journals.lww.com/hep/fulltext/2024/05000/aasld_practice_guidance_on_risk_stratification_and.22.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37870298?tool=bestpractice.com
Video capsule endoscopy is a safe and well-tolerated alternative for patients who are not candidates for EGD, or if EGD is not available.[39]McCarty TR, Afinogenova Y, Njei B. Use of wireless capsule endoscopy for the diagnosis and grading of esophageal varices in patients with portal hypertension: a systematic review and meta-analysis. J Clin Gastroenterol. 2017 Feb;51(2):174-82.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5218864
http://www.ncbi.nlm.nih.gov/pubmed/27548729?tool=bestpractice.com
However, sensitivity of capsule endoscopy is not sufficient to replace EGD as an initial exploration.[39]McCarty TR, Afinogenova Y, Njei B. Use of wireless capsule endoscopy for the diagnosis and grading of esophageal varices in patients with portal hypertension: a systematic review and meta-analysis. J Clin Gastroenterol. 2017 Feb;51(2):174-82.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5218864
http://www.ncbi.nlm.nih.gov/pubmed/27548729?tool=bestpractice.com
[40]Colli A, Gana JC, Turner D, et al. Capsule endoscopy for the diagnosis of oesophageal varices in people with chronic liver disease or portal vein thrombosis. Cochrane Database Syst Rev. 2014 Oct 1;(10):CD008760.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008760.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/25271409?tool=bestpractice.com
