Recommendations
Urgent
Start treatment immediately if a senior clinical decision-maker (e.g., ST3 level doctor in the UK) makes a diagnosis of suspected sepsis.[41][42] NHS England: Sepsis Opens in new window
PLUS and BaSICSSepsis is suspected based on acute deterioration (e.g., National Early Warning Score 2 [NEWS2] score of 5 or more, or a similar trigger using another validated scoring system) in a patient with known or likely infection.[41][42] For more detail on when to suspect sepsis, see Diagnosis recommendations.
Treat suspected sepsis (i.e., new organ dysfunction related to severe infection) promptly. Establish venous access early so you can start initial treatment according to the timeframes below:[3][41][45][47]
Within 1 hour of initial severity assessment for patients with a NEWS2 score of 7 or more calculated on initial assessment in the accident and emergency department or on ward deterioration (or with a score of 5 or 6 if there are additional clinical or carer concerns, continuing deterioration or lack of improvement, surgically remediable sepsis, neutropenia, or blood gas/laboratory evidence of organ dysfunction)
A patient is also at high risk of severe illness or death from sepsis if they have a NEWS2 score below 7 and a single parameter contributes 3 points to their NEWS2 score and a medical review has confirmed that they are at high risk.[3]
or
Within 3 hours for patients with a NEWS2 score of 5 or 6.
Start the following treatments:[3][41][47]
Intravenous antibiotics: where there is evidence of a bacterial infection, administer broad-spectrum empirical intravenous antibiotics before a pathogen is identified.[3] Only give antibiotics if they have not been given before for this episode of sepsis.[3]
Follow local policy and consider discussing with microbiology/infectious disease colleagues to determine the most appropriate choice; use a ‘start smart then focus’ approach[41][136]
Target the presumed site of infection if known
Take bloods immediately, preferably before antibiotics are started (although sampling should not delay the administration of antibiotics)[3][43][48][49]
Narrow the choice of antibiotic as soon as a pathogen has been identified and sensitivities are available[3][43][137]
Intravenous fluids: 500 mL of crystalloid, with sodium in the range 130 to 154 mmol/L (130 to 154 mEq/L), over less than 15 minutes, if either lactate is over 2 mmol/L or systolic blood pressure is less than 90 mmHg.[3][41][67]
Give this intravenous fluid bolus, if indicated, without delay (within 1 hour of identifying a patient is at high risk).[3]
Consider giving an intravenous fluid bolus to patients with a high risk of severe illness or death from sepsis if lactate is 2 mmol/L or lower.[3]
Repeat if clinically indicated
Do not exceed 30 mL/kg
Oxygen: as needed. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia.
Carry out the following investigations:
Blood cultures
Lactate level
Hourly urine output.
Consult local protocols for specific routes of escalation. In general, in hospital, if a patient is at high risk of severe illness or death from sepsis (e.g., NEWS2 score of 7 or more) or does not respond within 1 hour of any intervention (antibiotics/fluid resuscitation/oxygen):[3]
Ensure the senior clinical decision-maker attends in person, and
Refer to or discuss with a critical care consultant or team, and
Inform the responsible consultant.
Signs that the person is not responding to resuscitation include lack of improvement or worsening:[3]
Tachycardia
Level of consciousness
Blood pressure
Respiratory rate
Blood lactate
Urine output
Peripheral perfusion
Blood gases.
Consider alerting critical care immediately if the patient is acutely unwell and:
Has hypotension that does not respond to initial fluid resuscitation within 1 hour.[3]
Is likely to require central venous access and the initiation of inotropes or vasopressors[3]
Has any feature of septic shock
Has neutropenia
Is immunodeficient.
Bear in mind that some patients (e.g., those who are frail) may not be suitable for management in intensive care settings. Consider the patient’s preferences for future care and their baseline health including their resuscitation status when determining the limits of treatment. Use this to feed into a personalised care plan appropriate to the individual patient.[45]
Urgent: in the community
Refer for emergency medical care in hospital (usually by blue-light ambulance in the UK) any patient who is acutely ill with a suspected infection and is:[3]
Deemed to be at high risk of deterioration due to organ dysfunction, as measured by a formal risk stratification process such as NEWS2, which is recommended by NHS England and the UK National Institute for Health and Care Excellence (NICE).[41][43]
At risk of neutropenic sepsis. See Febrile neutropenia.[139]
Communicate your concern to the ambulance service and hospital colleagues by using the words 'suspected sepsis', and offer the outcome of a physiological assessment (e.g., NEWS2 score).[51]
Start oxygen therapy, if indicated, while awaiting the ambulance if resources are available to do so.[50]
Ensure you have a mechanism in place to administer antibiotics, if needed, to any high-risk patient (either at your practice or via the ambulance service) if the transfer to hospital is likely to be delayed.
Key Recommendations
Sepsis is a medical emergency.[3][43] The key to improving outcomes is early recognition and prompt treatment, as appropriate, of patients with suspected or confirmed infection who are deteriorating and at risk of organ dysfunction.[3][43]
Always use your clinical judgement.[41]
Take into account the full clinical picture of the individual patient in front of you including their NEWS2 score.
Identify and treat underlying source
Early and adequate source identification and control is critical. Undertake intensive efforts, including imaging, to attempt to identify the source of infection in all patients with sepsis.[3][43]
Consider the need for urgent source control as soon as the patient is stable.
The respiratory tract is the most common site of infection in most people with sepsis.[20][61] However, in people over age 65 years, the most common site is the genitourinary tract.[21][22]
Where organisms are identified, bacteria (gram-positive and gram-negative) are the causative organism in the majority of people with sepsis, with gram-positive bacterial and fungal infections increasing in frequency.[140]
Protocolised approaches
Your institution may use a guideline-based care bundle as an aide-memoire to ensure key interventions are carried out in a timely way as appropriate for the individual patient. Check local protocols for the recommended approach in your area. Examples include the following.
The Sepsis Six resuscitation bundle from the UK Sepsis Trust[47]
Sepsis Six is a practical checklist of interventions that must be completed within 1 hour of identifying a patient with suspected sepsis with a NEWS2 score of 7 or more or other features of critical illness (lactate >2 mmol/L (>18 mg/dL); chemotherapy in last 6 weeks; other organ failure evident [e.g., acute kidney injury]; patient looks extremely unwell; patient is actively deteriorating).[45][47] The original paper outlining this approach, published in 2011, remains the only published evidence on Sepsis Six and was subsequently contested.[56][57] The six interventions are:[47]
Inform a senior clinician
Give oxygen if required
Obtain intravenous access/take blood cultures
Give intravenous antibiotics
Give intravenous fluids
Monitor.
In 2022, the criteria for triggering the Sepsis Six bundle were aligned with UK Academy of Medical Royal Colleges guidance and in 2024 the bundle was updated to reflect updates to NICE guidance.[3][45][47]
The 2018 hour-1 care bundle from the Surviving Sepsis Campaign (SSC)[46]
The SSC proposes a 1-hour care bundle, based on the premise that the temporal nature of sepsis means benefit from even more rapid identification and intervention. The SSC identifies the start of the bundle as patient arrival at triage. It draws out five investigations and interventions to be initiated within the first hour:[46]
Measure lactate level and remeasure if the initial lactate level is greater than 2 mmol/L (18 mg/dL)
Obtain blood cultures before administration of antibiotics
Administer broad-spectrum intravenous antibiotics
Begin rapid administration of crystalloid at 30 mL/kg for hypotension or lactate level greater than or equal to 4 mmol/L (36 mg/dL)
Start vasopressors if the patient is hypotensive during or after fluid resuscitation to maintain mean arterial pressure level greater than or equal to 65 mmHg.
Subsequent guidance from the SSC and the UK Academy of Medical Royal Colleges supports a more nuanced approach to investigating and treating patients with suspected sepsis presenting with less severe illness (e.g., without septic shock, or NEWS2 score less than 7).[43][45] Although early identification and prompt, tailored treatment are key to the successful management of sepsis, none of the published protocolised approaches are supported by evidence.[58][59] Therefore, your clinical judgement is a key part of any approach.[41]
Reassess and monitor
Ensure frequent reassessment of the patient’s haemodynamic status throughout the initial resuscitation period. Make sure any patient with suspected sepsis has frequent and ongoing monitoring (e.g., using an early warning score such as NEWS2).[3]
Depending on the facilities available, consider continuous monitoring, or a minimum of once every 30 minutes.[3]
Include:
Oxygen saturation
Respiratory rate
Heart rate
Blood pressure
Temperature
Hourly fluid balance (including urine output)
Lactate level.
Consider using a validated scale such as the Glasgow Coma Scale or the AVPU ('Alert, responds to Voice, responds to Pain, Unresponsive') scale to monitor the mental state of a patient with suspected sepsis. [ Glasgow Coma Scale Opens in new window ] [3]
Be aware that a patient with a NEWS2 score of less than 5 might also have or develop sepsis. In this group, continue to be aware of the risk of sepsis and specifically look for indicators that suggest the possibility of underlying infection and sepsis:[3][41]
A single NEWS parameter of 3 or more
Non-blanching petechial or purpuric rash/mottled/ashen/cyanotic skin
Responds only to voice or pain, or unresponsive
Not passed urine in last 18 hours or urine output <0.5 mL/kg/hour
Lactate ≥2 mmol/L (≥18 mg/dL).
If a single parameter contributes 3 points to a patient’s NEWS2 score, request a high-priority review by a clinician with core competencies in the care of acutely ill patients (in the UK, FY2 or above), for a definite decision on the person's level of risk of severe illness or death from sepsis.[3] A single parameter contributing 3 points to a NEWS2 score is an important red flag suggesting an increased risk of organ dysfunction and further deterioration.[3] Clinical judgement is required to evaluate whether the patient’s condition needs to be managed as per a higher risk level than that suggested by their NEWS2 score alone. A patient’s risk level should be re-evaluated each time new observations are made or when there is deterioration or an unexpected change.[3]
The overarching goals are to:
Resuscitate the patient and restore haemodynamic stability using supportive measures to correct hypoxaemia, hypotension, and impaired tissue oxygenation (hypoperfusion)
Rapidly identify the source of infection; contain and treat
Where there is evidence of a bacterial infection, start effective broad-spectrum intravenous antibiotics:[3][41][45]
Within 1 hour of the risk being recognised in patients who are critically ill (including those with septic shock, sepsis associated with rapid deterioration, or a NEWS2 score ≥7 on initial assessment in the accident and emergency department or on ward deterioration).
Within 3 hours in patients with suspected sepsis with less severe illness (e.g., NEWS2 score of 5 or 6).
Switch to a targeted antibiotic once the pathogen has been confirmed[3]
Maintain organ system function, guided by cardiovascular monitoring, and interrupt the progression of organ failure.
Early recognition of sepsis is critically important, but this can be challenging as patients often present with subtle and/or non-specific signs.[141]
In practice, you should make a diagnosis of suspected sepsis and start immediate treatment if the patient is acutely unwell and meets both of the following criteria:[3][41]
Signs or symptoms suggestive of infection are present
AND
Your clinical assessment of the patient indicates a risk of deterioration due to organ dysfunction.
Always use your clinical judgement when assessing the risk of deterioration due to sepsis, alongside a systematic approach to assessing vital observations.[3][41] Consult local guidelines for the recommended approach.
The National Early Warning Score 2 (NEWS2) is the most widely used early warning score in the UK National Health Service. NHS England: Sepsis Opens in new window
In hospital: use NEWS2 or an alternative early warning score.[41][42][44] NEWS2 is endorsed by NHS England and the National Institute for Health and Care Excellence (NICE) for use in this setting.[3][41] NEWS2 is also recommended for use in acute mental health settings and ambulances.[3]
NICE recommends to use NICE high-risk criteria for stratification of risk (rather than NEWS2) in an acute setting in patients who are or have recently been pregnant.[3]
In the community and in custodial settings: use an early warning score such as NEWS2, which is recommended by NHS England and supported by the Royal College of General Practitioners in the UK.[41][51] An alternative in the UK is to use the NICE high-risk criteria.[3]
None is validated in primary care.[51]
NHS England and the Royal College of Physicians in the UK set the threshold for starting immediate sepsis treatment as a NEWS2 score of 5 or more.[41][42]
You should strongly suspect sepsis and consider the need to start immediate treatment if the patient:
Has any single NEWS2 parameter score of 3 or more OR
Has a non-blanching rash or has mottled/ashen/cyanotic skin OR
Is unresponsive or only responds to voice or pain OR
Has not passed urine for 18 or more hours (or urine output <0.5 mL/kg/hour if catheterised) OR
Has a lactate level ≥2 mmol/L (≥18 mg/dL).[41]
The UK’s Academy of Medical Royal Colleges (AOMRC) stratifies the urgency of treatment for sepsis according to NEWS2 score. The AOMRC and NICE recommend:[3][45]
Initial treatment within 1 hour for a patient with a NEWS2 score of 7 or more calculated on initial assessment in the accident and emergency department or on ward deterioration (or if they meet other criteria, as per below)
A patient is also at high risk of severe illness or death from sepsis if they have a NEWS2 score below 7 and a single parameter contributes 3 points to their NEWS2 score and a medical review has confirmed that they are at high risk.[3]
Initial treatment within 3 hours for a patient with a NEWS2 score of 5 or 6
Interpreting the initial NEWS2 score in the context of clinical assessment, so that severity status and corresponding actions are upgraded to at least the next NEWS2 level if there is clinical or carer concern, continuing deterioration or lack of improvement, surgically remediable sepsis, neutropenia, or blood gas/laboratory evidence of organ dysfunction (including elevated serum lactate).
For full details of risk stratification, see Diagnosis recommendations.
Start treatment immediately if a senior clinical decision-maker (e.g., ST3 level doctor in the UK) makes a diagnosis of suspected sepsis, based on acute deterioration (e.g., National Early Warning Score 2 [NEWS2] score of 5 or more, or a similar trigger using another validated scoring system) in a patient with known or likely infection.[41][42][45]
Urgent actions
For any acutely ill and deteriorating patient with a suspected or known bacterial infection and suspected sepsis, above all else prioritise (if needed):[3][47]
Securing their airway
Correcting hypoxaemia
Establishing venous access for the early administration of antibiotics and fluids.
Demonstrates how to obtain femoral venous access.
Early and adequate source identification and control is critical. If your examination of the patient identifies a clear source of infection, consider the need for urgent source control, as soon as the patient is stable, particularly for:[43]
Gastrointestinal sources (such as visceral abscesses, cholangitis, or peritonitis secondary to perforation)
Severe skin infections (e.g., necrotising fasciitis)
Infection involving an indwelling device, where a procedure or surgery is likely to be required.
Give immediate, targeted intravenous antibiotics in people with sepsis thought to arise from a central nervous system source (e.g., suspected meningitis or meningococcal sepsis).[3]
Immediately give a third-generation cephalosporin such as ceftriaxone.
In community settings, pre-hospital administration of benzylpenicillin is recommended.
Follow local policy and consider discussing with microbiology/infectious disease colleagues to determine the most appropriate choice; use a ‘start smart then focus’ approach.[41][136]
Practical tip
If intravenous access is not feasible or is likely to lead to a delay in starting antibiotics and fluids, use intra-osseous access as an interim measure.
Intravenous antibiotics
Where there is evidence of a bacterial infection and sepsis is strongly suspected (based on acute deterioration [e.g., NEWS2 score of 5 or more, or a similar trigger using another validated scoring system]), give broad-spectrum intravenous antibiotics promptly.[3][41][43][47] Do this before a pathogen is identified but after blood cultures have been taken.[3][41][43][47] Only give antibiotics if they have not been given before for this episode of sepsis.[3]
The UK Academy of Medical Royal Colleges and NICE recommends administration of antimicrobials:[3][45]
Within 1 hour if the NEWS2 score is 7 or more calculated on initial assessment in the accident and emergency department or on ward deterioration, or with a score of 5 or 6 if there is clinical or carer concern, continuing deterioration or lack of improvement, surgically remediable sepsis, neutropenia, or blood gas/laboratory evidence of organ dysfunction (including elevated serum lactate)
If a patient has a NEWS2 score of 5 or 6 calculated on initial assessment in the accident and emergency department or on ward deterioration and a single parameter contributes 3 points to the total NEWS2 score, clinical judgement should be used to determine the likely cause of the 3 points in one parameter. If the likely cause is the current infection, manage as high risk and give broad-spectrum antibiotics within 1 hour of the NEWS2 score being calculated on initial assessment in the accident and emergency department or on ward deterioration.[3]
Within 3 hours if the NEWS2 score is 5 or 6.
The Surviving Sepsis Campaign international guideline recommends empirical combination therapy (using at least two antibiotics of different antimicrobial classes covering gram-negative bacilli) for patients at high risk of infection from multidrug resistant (MDR) organisms, particularly in those with septic shock.[43]
Follow local policy and consider discussing with microbiology/infectious disease colleagues to determine the most appropriate choice. Once a decision is made to give antibiotics, do not delay administration any further.[3]
More info: Antimicrobial resistance
NHS England recommends following a ‘start smart then focus’ approach for antibiotic use in people with sepsis.[41]This is derived from Public Health England guidance, which outlines an evidence-based approach to improving antimicrobial prescribing and stewardship in hospital settings.[136]The prevalence of antimicrobial resistance (AMR) has risen alarmingly over the last 50 years and no new classes of antibiotics have been developed in decades. By 2050 it is estimated that AMR will kill 10 million people per year, more than cancer and diabetes combined.[142]The relationship between antibiotic exposure and antibiotic resistance is unambiguous not only at the population level but also in individual patients.[143][144]
Start smart – in the context of sepsis:[136]
Do not start antimicrobial therapy unless there is clear evidence of infection
Take a thorough drug allergy history
Initiate prompt effective antibiotic treatment within 1 hour of diagnosis (or as soon as possible) in patients with sepsis who are critically ill (e.g., septic shock, sepsis associated with rapid deterioration, or NEWS2 score of 7 or more calculated on initial assessment in the accident and emergency department or on ward deterioration) or with life-threatening infections. Avoid inappropriate use of broad-spectrum antibiotics[3][45]
Comply with local antimicrobial prescribing guidance
Document clinical indication (and disease severity if appropriate), drug name, dose, and route on drug chart and in clinical notes Include review/stop date or duration
Obtain cultures prior to starting therapy where possible (but do not delay therapy).
Then focus – in the context of sepsis:[136]
Review the clinical diagnosis and the continuing need for antibiotics at 48 to 72 hours* and document in a clear plan of action – the ‘antimicrobial prescribing decision
The ‘antimicrobial prescribing decision’ options are:
Stop antibiotics if there is no evidence of infection
Switch antibiotics from intravenous to oral
Change antibiotics – ideally to a narrower spectrum, or broader if required
Continue and document next review date or stop date
It is essential that the review and subsequent decision is clearly documented in the clinical notes and on the drug chart where possible (e.g., ‘stop antibiotic’).
*In clinical practice, daily prompting about de-escalation is encouraged.
The UK’s Academy of Medical Royal Colleges recommends stratifying patients with suspected sepsis according to severity of illness at presentation, allowing a 3-hour window to investigate patients with less severe illness (e.g., NEWS2 score of 5 or 6). This should improve accuracy of treatment and help to reduce antimicrobial resistance.[45]
Target the presumed site of infection.[3][43]
If there is no clinical evidence to suggest a specific site of infection but a senior clinical decision-maker strongly suspects the presence of a bacterial infection, still give empirical broad-spectrum intravenous antibiotics.[41] Choose an empirical antibiotic based on:[145][146]
Local antibiotic protocols and resistance patterns
Consult microbiology/infectious disease colleagues to determine the most appropriate choice
The likely causative organism
The patient’s immune function.
Practical tip
Check local policies regarding repeat cultures. These are indicated particularly if there are persistent or repeated fever spikes or if you identify a potential new site of infection. Observations from studies to date support taking as many as four blood culture sets over a 24-hour period for >99% test sensitivity.[147]
Practical tip
If a patient has a mild allergy (e.g., rash) to an unknown antibiotic, you should still give empirical broad-spectrum antibiotics if indicated to prevent delays in the treatment of sepsis, which is likely to worsen outcome. If the antibiotic is known and is part of the empirical protocol for your hospital, discuss potential alternatives with a microbiologist.
Evidence: 1- and 3-hour antibiotic targets
There is widespread agreement that antibiotics should be offered to people with sepsis, within a timeframe that depends on their risk of severe illness or death. The UK National Institute for Health and Care Excellence (NICE), the Surviving Sepsis Campaign (SSC) and the UK Academy of Medical Royal Colleges (AOMRC) all recommend a 1-hour target time threshold for patients at high risk and a 3-hour target time threshold for patients at moderate risk.[43][45]
Two meta-analyses published in 2015 and 2020 compared outcomes for patients with sepsis and septic shock given either immediate (within 1 hour of onset) or early (between 1 and 3 hours from onset) antibiotics; neither meta-analysis identified a difference in mortality between these thresholds.[148][149] One 2021 systematic review (without meta-analysis) of 35 sepsis studies concluded that two-thirds of studies reported an association between early administration of antibiotic therapy and patient outcome, but there was widespread variation in metrics and no robust time thresholds emerged.[150] Almost all of the studies included in these reviews were observational.[148][149][150]
The 2021 update of the SSC guideline recommends that patients with possible sepsis without shock should receive a time-limited course of rapid investigation with administration of antimicrobials within 3 hours if there is persisting concern for infection.[43] Similarly, 2022 guidance from the UK’s AOMRC, based largely on the evidence above, recommends a 3-hour window for investigating and treating patients with less severe illness, while continuing to recommend a 1-hour target for treating patients with more severe illness (e.g., NEWS2 score of 7 or more, or septic shock). The recommended timeframes in the Sepsis Trust’s Sepsis Six bundle have since been adjusted in line with AOMRC guidance.[43][45][47]
At the 2024 update of the NICE ‘Suspected sepsis: recognition, diagnosis and early management’ guideline, the committee agreed by consensus to change NICE guidance to also align with the AOMRC guidance.[3]
In their discussion, NICE highlighted that the reason for the longer target time threshold for those at moderate risk was to give more time to establish the diagnosis and guide antibiotic choice, and that the 3-hour limit is a maximum not an aim.
In intensive care settings only, consider prolonged infusion when giving beta-lactam antibiotics to patients with sepsis (apart from those with kidney-related complications).[151] Note that prolonged infusion times are not licensed as most manufacturers advise infusion of beta-lactam antibiotics over 15 to 60 minutes.
Evidence: Prolonged antibiotic infusion
Intravenous antibiotics, administered over 3 hours, are linked to lower death rates in sepsis.[151]Prolonged infusion should be easy to apply in the intensive care setting, without the need for additional training or equipment.
A systematic review and meta-analysis pooled the results of 22 randomised controlled trials involving 1876 adults with sepsis. The trials compared prolonged versus short-term administration of any antipseudomonal beta-lactam. Carbapenems were studied in nine trials, penicillins in nine trials, and cephalosporins in eight trials.[151]
Prolonged infusion was associated with lower all-cause mortality than short-term infusion, with 13.6% deaths compared with 19.8% (risk ratio [RR] 0.70, 95% CI 0.56 to 0.87; 17 studies, 1597 participants).
There was no significant difference between prolonged and short-term infusion for clinical cure or improvement (RR 1.06, 95% CI 0.96 to 1.17; 11 studies, 1219 participants).
There was no difference in reported adverse events between the groups (RR 0.88, 95% CI 0.71 to 1.09; 7 studies, 980 participants).
Two trials had no incidence of antibiotic resistance, and two trials had no difference in resistance between the two methods of antibiotic administration (RR 0.60, 95% CI 0.15 to 2.38).
Intravenous fluids
Give 500 mL of crystalloid fluid, with a sodium content between 130 mmol/L and 154 mmol/L (130 to 154 mEq/L) (e.g., 0.9% sodium chloride or Hartmann’s solution), over less than 15 minutes to patients who need fluid resuscitation (if either lactate is over 2 mmol/L or systolic blood pressure is less than 90 mmHg).[3][41][67]
Give this intravenous fluid bolus, if indicated, without delay (within 1 hour of identifying a patient is at high risk).[3]
Consider giving an intravenous fluid bolus to patients with a high risk of severe illness or death from sepsis if lactate is 2 mmol/L or lower.[3]
Reassess the patient’s haemodynamic status after the first bolus to consider whether a second is required.[3] If there is no response to either the first or second bolus, ensure the senior clinical decision-maker attends in person.[3]
Intravenous fluid resuscitation may be lifesaving in patients with hypotension. This is because in sepsis there is vasodilation and capillary leakage, which means that patients can rapidly become intravascularly deplete.[3]
In patients with sepsis-induced hypoperfusion (as indicated by a systolic blood pressure <90 mmHg, a raised lactate level, or signs of organ dysfunction), the Surviving Sepsis Campaign international guideline recommends a total of at least 30 mL/kg of intravenous crystalloid over the first 3 hours.[43]
If the patient’s initial lactate level is raised, the guideline recommends serial lactate measurements to guide the need for further intravenous fluids (with the goal of normalising lactate levels).[43]
Practical tip
The delivery of appropriate rapid fluid challenges is intended to restore the imbalance between oxygen supply and demand to the tissues. Patients who do not respond to rapid delivery of adequate volumes of intravenous fluids are in septic shock and need immediate referral to critical care. The immediate priority in this group of patients is to restore the circulation and oxygen delivery.
Check local protocols for specific recommendations on fluid choice. There is debate, based on conflicting evidence, on whether there is a benefit in using normal saline or balanced crystalloid in critically ill patients.
Practical tip
Be aware that large volumes of normal saline as the sole fluid for resuscitation may lead to hyperchloraemic acidosis.
Also note that use of lactate-containing fluid in a patient with impaired liver metabolism may lead to a spuriously elevated lactate level, so results need to be interpreted with other markers of volume status.
Evidence: Choice of fluid
Evidence from two large randomised controlled trials (RCTs) suggests there is no difference between normal saline and a balanced crystalloid for critically ill patients in mortality at 90 days, although results from two meta-analyses including these RCTs point to a possible small benefit of balanced solutions compared with normal saline.
There has been extensive debate over the choice between normal saline (an unbalanced crystalloid) versus a balanced crystalloid (such as Hartmann’s solution [also known as Ringer’s lactate] or Plasma-Lyte®). Clinical practice varies widely, so you should check local protocols.
In 2021-2022 two large double-blind RCTs were published assessing intravenous fluid resuscitation in intensive care unit (ICU) patients with a balanced crystalloid solution (Plasma-Lyte) versus normal saline: the Plasma-Lyte 148 versus Saline (PLUS) trial (53 ICUs in Australia and New Zealand; N=5037) and the Balanced Solutions in Intensive Care Study (BaSICS) trial (75 ICUs in Brazil; N=11,052).[154][155]
In the PLUS study, 45.2% of patients were admitted to ICU directly from surgery (emergency or elective), 42.3% had sepsis, and 79.0% were receiving mechanical ventilation at the time of randomisation.
In BaSICS, almost half the patients (48.4%) were admitted to ICU after elective surgery and around 68% had some form of fluid resuscitation before being randomised.
Both found no difference in 90-day mortality overall or in prespecified subgroups for patients with acute kidney injury (AKI), sepsis, or post-surgery. They also found no difference in the risk of AKI.
In BaSICS, for patients with traumatic brain injury, there was a small decrease in 90-day mortality with normal saline; however, the overall number of patients was small (<5% of total included in the study) so there is some uncertainty about this result. Patients with traumatic brain injury were excluded from PLUS as the authors felt these patients should be receiving saline or a solution of similar tonicity.
A meta-analysis of 13 RCTs (including PLUS and BaSICS) confirmed no overall difference, although the authors did highlight a non-significant trend towards a benefit of balanced solutions for risk of death.[156][Evidence A]
A subsequent individual patient data meta-analysis included 6 RCTs of which only PLUS and BaSICS were assessed as being at low risk of bias. There was no statistically significant difference in in-hospital mortality (odds ratio [OR] 0.96, 95% CI 0.91 to 1.02). However, the authors argued that using a Bayesian analysis there was a high probability that balanced solutions reduced in-hospital mortality, although they acknowledged that the absolute risk reduction was small.[157]
A pre-specified sub-group analysis of patients with traumatic brain injury (N=1961) found that balanced solutions increased the risk of in-hospital mortality compared with normal saline (OR 1.42, 95% CI 1.10 to 1.82).
Previous evidence has been mixed.
One 2015 double-blind, cluster randomised, double-crossover trial conducted in 4 ICUs in New Zealand (N=2278), the 0.9% Saline vs Plasma-Lyte® for ICU fluid Therapy (SPLIT) trial, found no difference for in-hospital mortality, AKI, or use of renal-replacement therapy.[158]
However, a 2018 US multicentre unblinded cluster-randomised trial - the isotonic Solutions and Major Adverse Renal events Trial (SMART), among 15,802 critically ill adults receiving ICU care - found possible small benefits from balanced crystalloid (Ringer’s lactate or Plasma-Lyte®) compared with normal saline. The 30-day outcomes showed a non-significant reduced mortality in the balanced crystalloid group versus the normal saline group (10.3% vs. 11.1%; odds ratio [OR] 0.90, 95% CI 0.80 to 1.01) and a major adverse kidney event rate of 14.3% versus 15.4%, respectively (OR 0.91, 95% CI 0.84 to 0.99).[159]
One 2019 Cochrane review included 21 RCTs (N=20,213) assessing balanced crystalloids versus normal saline for resuscitation or maintenance in a critical care setting.[160]
The 3 largest RCTs in the Cochrane review (including SMART and SPLIT) all examined fluid resuscitation in adults and made up 94.2% of participants (N=19,054).
There was no difference in in‐hospital mortality (OR 0.91, 95% CI 0.83 to 1.01; high-quality evidence as assessed by GRADE), acute renal injury (OR 0.92, 95% CI 0.84 to 1.00; GRADE low), or organ system dysfunction (OR 0.80, 95% CI 0.40 to 1.61; GRADE very low).
The Surviving Sepsis Campaign 2021 guideline update makes a weak recommendation (low-quality evidence) in favour of administering a balanced crystalloid (such as Hartmann's solution [Ringer's lactate] or Plasma-Lyte®) to patients with sepsis, based on evidence published prior to the BaSICS trial results.
Subgroup analysis of patients with sepsis within the BaSICS trial showed no difference in 90-day mortality between patients given normal saline (an unbalanced crystalloid) versus a balanced crystalloid. However, the authors comment that the subgroup analysis should be considered as hypothesis-generating only.[154] Further RCTs are awaited.
Practical tip
To guide the need for further intravenous fluids, it can sometimes be helpful to use bedside ultrasound to monitor changes in inferior vena cava (IVC) diameter during respiration.[161][162]
In the spontaneously breathing patient: consider additional fluid resuscitation if there is a collapsed (or collapsing) IVC.
In the mechanically ventilated patient: an increase in IVC size >18% (or visible to the naked eye) with positive pressure ventilation suggests fluid-responsiveness.
Practical tip
Use the passive leg-raising test to predict fluid-responsiveness if adequate monitoring is available.[67][163]
This is a useful indicator of fluid-responsiveness, which should be assessed using devices that can continuously monitor cardiac output in real time (e.g., Pulse index Continuous Cardiac Output [PiCCO] monitor or oesophageal Doppler), usually in an intensive care unit rather than a general ward setting.
Sit the patient upright at 45° and tilt the entire bed through 45°.
Patients with a positive test have a >10% increase in cardiac output or stroke volume, indicating more fluids may be required.
The passive leg-raise response may be misleading in conscious patients who are uncomfortable or in pain when lying flat.
Oxygen
Monitor controlled oxygen therapy. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia.
Evidence suggests that liberal use of supplemental oxygen (target SpO2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[138]
A lower target SpO2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[50]
Evidence: Target oxygen saturation in acutely ill adults
Too much supplemental oxygen increases mortality.
Evidence from a large systematic review and meta-analysis supports conservative/controlled oxygen therapy versus liberal oxygen therapy in acutely ill adults who are not at risk of hypercapnia.
Guidelines differ in their recommendations on target oxygen saturation in acutely unwell adults who are receiving supplemental oxygen.
The 2017 British Thoracic Society (BTS) guideline recommends a target SpO2 range of 94% to 98% for patients not at risk of hypercapnia, whereas the 2022 Thoracic Society of Australia and New Zealand (TSANZ) guideline recommends 92% to 96%.[50][164]
The 2022 Global Initiative For Asthma (GINA) guidelines recommend a target SpO2 range of 93% to 96% in the context of acute asthma exacerbations.[165]
One systematic review including a meta-analysis of data from 25 randomised controlled trials published in 2018 found that in adults with acute illness, liberal oxygen therapy (broadly equivalent to a target saturation >96%) is associated with higher mortality than conservative oxygen therapy (broadly equivalent to a target saturation ≤96%).[138] In-hospital mortality was 11 per 1000 higher for the liberal oxygen therapy group versus the conservative therapy group (95% CI 2 to 22 per 1000 more). Mortality at 30 days was also higher in the group who had received liberal oxygen (relative risk 1.14, 95% CI 1.01 to 1.29). The trials included adults with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery. Studies that were limited to people with chronic respiratory illness or psychiatric illness, or patients on extracorporeal life support, receiving hyperbaric oxygen therapy, or having elective surgery, were all excluded from the review.
An upper SpO2 limit of 96% is therefore reasonable when administering supplemental oxygen to patients with acute illness who are not at risk of hypercapnia. However, a higher target may be appropriate for some specific conditions (e.g., pneumothorax, carbon monoxide poisoning, cluster headache, or sickle cell crisis).[166]
In 2019 the BTS reviewed its guidance in response to this systematic review and meta-analysis and decided an interim update was not required.[167]
The committee noted that the systematic review supported the use of controlled oxygen therapy to a target.
While the systematic review showed an association between higher oxygen saturations and higher mortality, the BTS committee felt the review was not definitive on what the optimal target range should be. The suggested range of 94% to 96% in the review was based on the lower 95% confidence interval and the median baseline SpO2 from the liberal oxygen groups, along with the earlier 2015 TSANZ guideline recommendation.
Subsequently, experience during the COVID-19 pandemic has also made clinicians more aware of the feasibility of permissive hypoxaemia.[168] The BTS guidance is due for a review in 2022.
Management of oxygen therapy in patients in intensive care is specialised and informed by further evidence (not covered in this summary) that is more specific to this setting.[169][170][171]
There is no specific evidence to show that giving oxygen improves clinical outcomes in sepsis. However, respiratory failure will lead to tissue hypoxia and anaerobic respiration. This is likely to lead to acidosis and consequently a poorer outcome.[172]
Ensure frequent and ongoing monitoring.[3]
Standard monitoring of vital signs, pulse oximetry, level of consciousness, and urinary output is important for any patient with suspected sepsis.
The National Institute for Health and Care Excellence (NICE) in the UK recommends continuous or half-hourly monitoring (depending on setting) for any patient considered to be at high risk of deterioration (e.g., using an early warning score such as NEWS2).[3]
For more information, see Risk stratification under Diagnosis recommendations.
Use a track-and-trigger scoring system such as the National Early Warning Score 2 (NEWS2) to identify any signs of deterioration.[3] Your monitoring should include:
Vital signs: heart rate, blood pressure, oxygen saturations, respiratory rate, and temperature
Measure blood pressure via an arterial line if the patient does not respond to initial treatment or needs vasoactive drugs. It provides precise, continuous monitoring, and access for arterial blood sampling
Lactate
The lactate level should decrease if the patient is clinically improving
Frequency of repeat lactate measurement depends on cause of sepsis and treatment given.
In the UK, use physiological track-and-trigger systems to monitor all adult patients in acute hospital settings.[3]
Consider using a validated scale such as the Glasgow Coma Scale or AVPU ('Alert, responds to Voice, responds to Pain, Unresponsive') scale to monitor the mental state of a patient with suspected sepsis . [ Glasgow Coma Scale Opens in new window ] [3]
Practical tip
AVPU should raise concerns if the assessment shows the patient is anything other than 'alert'.
Any patient with sepsis may be at significant risk of severe illness or death so it is vital to consider escalation of care to senior colleagues and/or healthcare facilities where increased and more advanced monitoring can be given (e.g., high-dependency unit/intensive care unit).[7][173][174]
Bear in mind that some patients (e.g., those who are frail) may not be suitable for management in intensive care settings. Consider the patient’s preferences for future care and their baseline health including their resuscitation status when determining the limits of treatment. Use this to feed into a personalised care plan appropriate to the individual patient.[45]
Consult local protocols for specific escalation routes but in general:
Ensure urgent review by a senior clinician (e.g., ST3 level doctor or higher in the UK) of any patient with a National Early Warning Score 2 (NEWS2) score of 5 or more calculated on initial assessment in the accident and emergency department or on ward deterioration:[3][41][45]
Within 30 minutes of initial severity assessment for any patient with an aggregate NEWS2 score of 7 or more; or with a NEWS2 score of 5 or 6 if there is clinical or carer concern, continuing deterioration or lack of improvement, surgically remediable sepsis, neutropenia, or blood gas/laboratory evidence of organ dysfunction (including elevated serum lactate - see below)
Within 1 hour of initial severity assessment for any patient with an aggregate NEWS2 score of 5 or 6.
Inform the responsible consultant if a patient is at high risk of severe illness or death from sepsis (e.g., NEWS2 score of 7 or more) does not respond within 1 hour of any intervention (antibiotics/fluid resuscitation/oxygen).[3] Ensure the senior clinical decision-maker attends in person.
Signs that the person is not responding to resuscitation include lack of improvement or worsening:[3]
Tachycardia
Level of consciousness
Blood pressure
Respiratory rate
Blood lactate
Urine output
Peripheral perfusion
Blood gases.
Consider alerting critical care immediately if the patient is acutely unwell and:
Is likely to require central venous access and the initiation of inotropes or vasopressors[3]
This includes any patient with evidence of circulatory dysfunction or shock, or those who do not respond to initial therapy (as outlined above) including initial fluid resuscitation within 1 hour.
Has any feature of septic shock
See Shock.
Has neutropenia
See Febrile neutropenia.
Is immunodeficient
Practical tip
Ensure a clear escalation plan has been discussed and agreed with the clinical team; include specific points of contact for nursing staff if you are leaving a patient for later review.
Involve a senior colleague and/or consider transferring to critical care sooner rather than later if the patient is not improving, or deemed high-risk. Examples include if the patient:
Is not responding to fluids
Needs inotropic support
Has a low Glasgow Coma Scale score
Needs ventilatory support.
Make intensive efforts to identify the anatomical source of infection as soon as possible.[3][43] Consider the need for urgent source control as soon as the patient is stable.
Start with a thorough and focused clinical history and examination, as well as initial investigations including imaging.[3]
Consider all lines and catheters as potential sources. Remove lines where appropriate.[43]
Assume that any intravenous route is likely to either be the source of the infection, or will seed infections in the bloodstream, making eradication particularly difficult. Therefore, the priority for source control is often to remove any intravenous devices after alternative vascular access has been obtained.[43]
Involve the relevant surgical team early on if surgical or radiological intervention is suitable for the source of infection.[3][45] The surgical team or interventional radiologist should seek senior advice about the timing of intervention and carry the intervention out as soon as possible, in line with the advice received.[3]
In practice, this may mean early transfer of the patient to a surgical centre if there are no facilities at your hospital.
Switch to a targeted antibiotic as soon as culture and sensitivity results are available and a pathogen has been identified.[43][137]
Tailor antibiotics based on source
Once a definitive source has been identified, if appropriate to continue treating the patient with antibiotics, choose a treatment regimen in line with local or national policy (which will take into account specialist knowledge of resistance patterns).[43][137] Also consider discussing with microbiology/infectious disease colleagues to determine the most appropriate choice.
Respiratory
Ensure treatment regimens cover common respiratory pathogens and atypical organisms such as Legionella pneumophila.
Abdominal
Ensure gram-positive and gram-negative organisms including anaerobes are covered.[175]
Arrange urgent surgical drainage or percutaneous drainage (where appropriate) for peritonitis or intra-peritoneal abscesses.[176]
Urinary tract
Ensure gram-negative coliforms and Pseudomonas are covered. Ensuring patency of the urinary tract is vital.
In people older than 65 years of age, genitourinary tract infections are the most common cause of sepsis.[21][22]
Soft tissue and joint
Includes septic arthritis, wound infections, cellulitis, and acute super-infections arising from chronic ulceration. Most infections are polymicrobial. Ensure gram-positive and gram-negative organisms including anaerobes are covered.
Beware necrotising fasciitis, which requires immediate surgical intervention (as does septic arthritis).
Practical tip
Necrotising fasciitis is notoriously difficult to diagnose. The initial symptoms are non-specific and the clinical course is often slower than might be expected. Typically, the first sign is pain disproportionate to the clinical findings, followed or accompanied by fever.[74]
Central nervous system
Relatively uncommon but potentially devastating source of sepsis. Beware meningococcal sepsis, which can be extremely rapidly fatal; if survived, can lead to greater morbidity than other forms of sepsis.
Give immediate, targeted antibiotics in people with sepsis thought to arise from a central nervous system source.[3]
Immediately give a third-generation cephalosporin, such as ceftriaxone, for suspected meningitis or meningococcal sepsis.[3]
In community settings, give benzylpenicillin before referring to hospital.[3]
Unknown or unclear
Continue broad-spectrum coverage to include all common pathogens if the source is unknown or unclear.[3]
Bear in mind that a definite source of infection cannot be found in 20% to 30% of people with sepsis.[9]
For any patient with suspected sepsis, consider the need for referral to a high-dependency unit for management by the critical care team.[177][178]
Refer to critical care as soon as possible any patient who does not respond to initial therapy, and in particular anyone:
With hypotension that does not respond to initial fluid resuscitation within 1 hour.[3]
Who is likely to require central venous access and initiation of inotropes or vasopressors[3]
With any feature of septic shock
See Shock
With neutropenia
Who is immunodeficient.
The following interventions should only be initiated by experienced members of the critical care team:[179]
Glycaemic control
Vasoactive drugs (vasopressors/inotropes)
Corticosteroids.
Additional intensive care measures that will be considered include:[43][180][181]
Stress ulcer prophylaxis (in people at risk of gastrointestinal bleeding)
With an H2 antagonist or proton-pump inhibitor
Deep venous thrombosis prophylaxis
With heparin and compression stockings
Enteral or parenteral nutrition
Administration of human albumin solution 4% to 5% in patients with sepsis and shock[3][43] who have not responded to substantial volumes of crystalloids
Transfusion of packed cells
Consult local protocols for recommended threshold
The Surviving Sepsis Campaign recommends using a threshold of 70 g/L (7 g/dL).[43]
Evidence: Threshold for transfusion of packed cells
In the general critical care population there is no improvement with blood transfusions given at a higher haemoglobin threshold compared with a lower haemoglobin threshold. Overall, a more restrictive transfusion strategy is recommended; however, individual patient factors should be taken into account.
The 2021 Surviving Sepsis Campaign guideline recommends a restrictive transfusion strategy for adults with sepsis or septic shock.[43] The guideline identifies the following evidence.
One multicentre parallel group randomised controlled trial (RCT) in people >16 years of age with septic shock (N=998) compared blood transfusion at a lower haemoglobin threshold with a higher threshold.[182]
There was no difference in 90-day mortality between groups (risk ratio [RR] 0.94, 95% CI 0.78 to 1.09).
The results were similar using different methods of analysis (adjusted for risk factors at baseline, and per-protocol analyses).
Ischaemic events, severe adverse reactions, and need for life support were also similar.
A second multicentre RCT (838 critically ill adults) compared a restrictive strategy of red-cell transfusion with a liberal strategy.[183]
Overall, 30-day mortality was similar in the two groups (18.7% vs. 23.3%, P = 0.11). Results were similar in the subgroup of patients with sepsis or septic shock (N=218) (22.8% vs. 29.7%, P = 0.36).
The 30-day mortality rates were significantly lower in patients who were less acutely ill and in patients younger than 55 years old with the restrictive transfusion strategy. However, this was not the case in those with clinically significant cardiac disease.
The mortality rate during hospitalisation was significantly lower in the restrictive-strategy group (22.3% vs. 28.1%, P = 0.05).
A single-centre RCT in critically ill adult cancer patients with septic shock (N=300) also compared a liberal strategy with a restrictive strategy.[184]
28-day mortality was less in the liberal group, although this difference was not statistically significant (45% vs. 56%, hazard ratio [HR] 0.74, 95% CI 0.53 to 1.04). However, 90-day mortality was significantly reduced in the liberal group (HR 0.72, 95% CI 0.53 to 0.97).
There was no difference in duration of intensive care or hospital stay between groups.
Meta-analysis of the three studies found no difference in 28-day mortality (odds ratio 0.99, 95% CI 0.67 to 1.46, quality of evidence as assessed by GRADE moderate).[43]
The guideline concluded that the evidence did not favour one strategy over the other. The authors therefore based their recommendation on resource use, cost-effectiveness, and health equity concerns.
There may be a case to consider giving transfusions at a higher haemoglobin level in some patients (e.g., those with myocardial ischaemia, severe hypoxaemia, or acute haemorrhage.[43]
In the initial resuscitative phase, transfusion to achieve a higher haematocrit of ≥30% may be appropriate.[177]
In patients requiring prolonged ventilatory support, give lung-protective ventilation using minimal peak inspiratory pressures (<30 cm H 2O) and permissive hypercapnia to specifically limit pulmonary compromise .[185]
Titrate fraction of inspired oxygen (FiO 2) to lowest effective levels to prevent oxygen toxicity and maintain central venous oxygen tension.
Glycaemic control
Although patients with sepsis are often hyperglycaemic, the optimal glucose target is unknown.
The Surviving Sepsis Campaign guideline recommends targeting a blood glucose level <10.0 mmol/L (<180 mg/dL).[43]
The National Institute for Health and Care Excellence (NICE) in the UK makes no recommendations on glycaemic control in sepsis.[3]
Evidence: Glycaemic control
Recent years have seen a shift in opinion and practice regarding glycaemic control in critically ill people. Since 2001, the use of tight glycaemic control has been advocated in people with sepsis. More recent evidence, however, suggests an increase in adverse events (e.g., severe hypoglycaemia) in patients managed with very tight glycaemic control (targeting a blood glucose below 6.1 mmol/L [110 mg/dL]).[186][187]The conflicting evidence has led to variations in recommendations in different countries and settings. Follow your local protocol.
An international randomised controlled trial (RCT) of 6104 critically ill medical and surgical patients found increased 90-day mortality (odds ratio 1.14, 95% CI 1.02 to 1.28) with tighter glucose control, possibly due to more frequent episodes of hypoglycaemia.[188]
A 2010 systematic review of 6 RCTs and a meta-analysis investigating tight glucose control (4.4 to 6.1 mmol/L [80-110 mg/dL]) versus less strict glucose control in critically ill patients in the intensive care unit setting found no significant improvement in mortality with tight glucose control, but it was associated with significantly more hypoglycaemic episodes compared with less strict glucose control.[189]
An RCT of critically ill patients in a primarily surgical intensive care setting found lower patient mortality with tight glucose control, 4.4 to 6.1 mmol/L (80-110 mg/dL), compared with ‘conventional’ more liberal glucose control.[190]
Vasoactive drugs
Selection of appropriate vasoactive agents should only take place under critical care supervision and may vary according to clinician preference and local practice guidelines.
Vasopressors for persistent haemodynamic instability
Vasopressors are used in a critical care setting to maintain a mean arterial pressure (MAP) ≥65 mmHg if the patient is unresponsive to fluid resuscitation.[43][179]
Failure to respond to initial fluid resuscitation is a sign of septic shock.[1]
Noradrenaline (norepinephrine) is the vasopressor of choice, mainly because it increases MAP.[43]
Practical tip
Vasopressors are usually administered via central venous access due to concerns of extravasation and tissue ischaemia. However, the Surviving Sepsis Campaign supports short-term (less than 6 hours) peripheral administration of vasopressors in a vein proximal to the antecubital fossa, depending on local availability, and expertise in placement, of central venous catheters.[43] Central venous access should be secured as soon as possible.
These patients should also have an arterial catheter inserted as soon as possible to ensure more accurate monitoring of arterial blood pressure.[43]
Evidence: Choice of vasopressor
Although a systematic review of 23 randomised trials of patients with shock found no convincing evidence for the superiority of one vasopressor over another,[191]more recent meta-analyses reported a higher mortality associated with dopamine than with noradrenaline.[192]
Inotropes
Inotropes can be considered for patients with low cardiac output despite adequate fluid resuscitation and vasopressor therapy.[3][43]
The Surviving Sepsis Campaign guideline recommends either adding dobutamine to noradrenaline or using adrenaline (epinephrine) alone for people with persistent hypoperfusion despite adequate volume status and arterial blood pressure.[43]
NICE makes no specific recommendations on inotrope selection in patients with sepsis.[3]
Practical tip
Suspect low cardiac output if the clinical examination reveals prolonged capillary refill times, low urine output, or poor peripheral perfusion. Confirm with cardiac output monitoring or by sampling central venous or pulmonary arterial blood to measure oxygen saturations.
When using inotropes, keep the patient’s heart rate at less than 100 beats per minute to minimise myocardial ischaemia.[179]
Corticosteroids
The Surviving Sepsis Campaign guideline recommends intravenous hydrocortisone for patients with an ongoing requirement for vasopressor therapy.[43]
NICE does not give any recommendations on the use of corticosteroids for managing sepsis in adults.[3]
Evidence: Benefits and harms of corticosteroids
In adults with sepsis, intravenous low-dose corticosteroids may reduce organ failure at 7 days, and duration of mechanical ventilation, vasopressor therapy, and intensive care stay. However, whether corticosteroids reduce short or longer-term mortality is unclear. Possible harms include an increased risk of neuromuscular weakness, hyperglycaemia, and hypernatraemia with corticosteroids compared with no corticosteroids.
The Surviving Sepsis Campaign (SSC) 2021 guideline made a weak recommendation for using intravenous low-dose corticosteroids for adults with septic shock and an ongoing need for vasopressors (overall evidence assessed as moderate using GRADE). This was a slight change from the prior 2016 recommendation due to the publication of three subsequent randomised controlled trials (VANISH, ADRENAL, and APROCHSS) and a meta-analysis including these studies (22 RCTs, N=7297).[43][193]
Duration of shock was reduced in patients who received corticosteroids compared with placebo (mean difference -1.52 days, 95% CI -1.71 to -1.32 days, quality of evidence as assessed by GRADE moderate).
Corticosteroids also reduced organ failure at 1 week, duration of mechanical ventilation, and intensive care stay, and increased vasopressor-free days.
However, there was no difference in short-term mortality (risk ratio [RR] 0.96, 95% CI 0.91 to 1.02, GRADE high) with similar results for longer-term mortality (RR 0.96, 95% CI 0.90 to 1.02, GRADE moderate).
Corticosteroid use possibly increased neuromuscular weakness (RR 1.21, 95% CI 1.01 to 1.45, GRADE low).
Corticosteroids also increased the risk of any adverse event but there was considerable heterogeneity.
No trials reported quality-of-life outcomes.
The guideline also noted that uncertainties remain about the optimal dose, timing of initiation, and duration of treatment.
Other systematic reviews have considered low-dose corticosteroids in adults and children with sepsis ( with or without shock). They have included slightly different studies and come to slightly different conclusions, particularly about mortality.
A Cochrane review (search date July 2019) included 61 trials (12,192 participants, 53 trials in adults only). There were no new studies comparing low-dose corticosteroids with placebo since ADRENAL and APROCHSS.[194]
2-day, 90-day, and hospital mortality were reduced with use of corticosteroids (GRADE moderate). However, there was no difference in mortality at 6 months to 1 year (GRADE low).
Intensive care and hospital length of stay were significantly reduced with corticosteroids (GRADE high).
Corticosteroids increased the risk of hypernatraemia (GRADE high) and probably increased the risk of hyperglycaemia (GRADE moderate). They also increased the risk of muscle weakness (GRADE high). They did not seem to increase the risk of superinfection (GRADE moderate).
There was no significant difference in gastroduodenal bleeding, stroke, cardiac events, or neuropsychiatric events.
A rapid clinical practice guideline was published in 2018 triggered by publication of ADRENAL and APROCHSS.[195] The panel made a weak recommendation for the use of corticosteroids in adults and children with sepsis ( with and without shock). This guideline was also underpinned by a systematic review (42 RCTs, N=10,194).[196]
The guideline panel concluded that it was uncertain whether corticosteroids reduced short-term mortality at 28 to 31 days (1.8% absolute risk reduction, 95% CI, 4.1% reduction to 0.8% increase, GRADE low), although they did seem to reduce longer-term mortality at 60 days to 1 year (2.2% absolute risk reduction; 95% CI, 4.1% reduction to 0%, GRADE moderate).
Other results were similar to those of the SSC 2021 guideline and the Cochrane systematic review.
[Figure caption and citation for the preceding image starts]: BMJ Rapid Recommendations: intravenous corticosteroids plus usual care versus usual care onlyLamontagne F, et al. BMJ 2018;362:k3284 [Citation ends].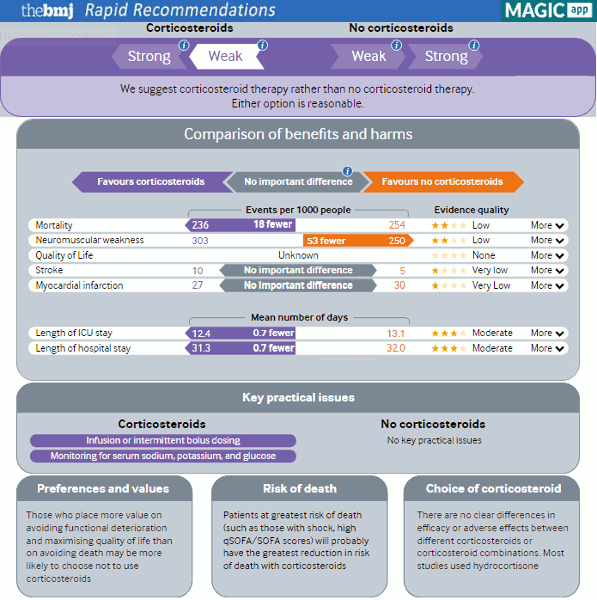
Antibiotics
Narrow choice of antibiotic as soon as a pathogen has been identified and sensitivities are available.[43][137] Assess the need to de-escalate antimicrobial therapy daily.[43]
Studies have shown that daily prompting about antimicrobial de-escalation is effective and may be associated with improved outcomes.[197][198]
Use the shortest effective course of antibiotics.[199]
Unnecessarily prolonged antibiotic treatment is associated with resistance. See More info: Antimicrobial resistance in Prompt management for all patients with suspected sepsis above.
Consult local microbiology guidance for other specific recommendations on de-escalation.
Most protocols will recommend switching from intravenous to oral antibiotics as soon as possible.
The Surviving Sepsis Campaign (SSC) recommends shorter over longer courses of antibiotics for patients with an initial diagnosis of sepsis or septic shock and adequate source control.[43] The optimal duration of antibiotic treatment in patients with sepsis remains contentious, with concerns regarding not only under-treatment but also the potential encouragement of antibiotic resistance. Consider seeking advice from microbiology/infectious disease colleagues.
Baseline serum procalcitonin is increasingly being used in critical care settings to guide decisions on how long to continue antibiotic therapy.[43][103][104][105]
Procalcitonin is a peptide precursor of calcitonin, which is responsible for calcium homeostasis.
The SSC suggests using procalcitonin alongside clinical evaluation to decide when to discontinue antimicrobials.[43]
Serum lactate
Measure serum lactate, on a blood gas, to monitor response to treatment.[43][47]
Lactate is a marker of stress and may be a marker of a worse prognosis (as a reflection of the degree of stress).
Raised serum lactate highlights the possibility of tissue hypoperfusion and may be present in many conditions.[75][76]
Lactate may normalise quickly after fluid resuscitation. Patients whose lactate levels fail to normalise after adequate fluids are the group that fare worst.
Lactate >4 mmol/L (>36 mg/dL) is associated with worse outcomes.
One study found in-hospital mortality rates as follows:[77]
Lactate <2 mmol/L (<18 mg/dL): 15%
Lactate 2.1 to 3.9 mmol/L (19 to 35 mg/dL): 25%
Lactate >4 mmol/L (>36 mg/dL): 38%.
Do not be falsely reassured by a normal lactate (<2 mmol/L [<18 mg/dL]).
This does not rule out the patient being acutely unwell or at risk of deterioration or death due to organ dysfunction. Lactate helps to provide an overall picture of a patient's prognosis but you must take into account the full clinical picture of the individual patient in front of you including their National Early Warning Score 2 (NEWS2) score to determine when/whether to escalate treatment.[3]
Practical tip
Lactate is typically measured using a blood gas analyser, although laboratory analysis can also be performed.
Traditionally, arterial blood gas has been recommended as the ideal means of measuring lactate accurately. However, in the emergency department setting it is more practical and quicker to use venous blood gas, which is recommended by NICE.[3] Evidence suggests good agreement at lactate levels <2 mmol/L (<18 mg/dL) with small disparities at higher lactate levels.[78][79][80]
Referring to hospital
Use your clinical judgement supported by physiological assessment.[51] Use National Early Warning Score 2 (NEWS2) scoring (encouraged by NHS England) to refer urgently to hospital any acutely unwell patient with suspected or confirmed infection according to the following triggers:[41] NHS England: Sepsis Opens in new window
Score 7 or more
Make an emergency referral to hospital (via blue-light ambulance) for immediate critical care input
Score 5-6 total, or 3 or more on any single parameter
Make an immediate referral to an acute care setting and ensure the patient is reviewed by an acute clinician within an hour.
Alternatively, refer for emergency medical care in hospital (usually by blue-light ambulance in the UK) any acutely unwell patient with suspected or confirmed infection who:[3]
Meets one or more of the UK National Institute for Health and Care Excellence (NICE) high-risk criteria (red flags)
Objective evidence of new altered mental state (e.g., new deterioration in Glasgow Coma Scale/AVPU ['Alert, responds to Voice, responds to Pain, Unresponsive'] scale)
Respiratory rate: ≥25 breaths per minute OR new need for oxygen (40% or more fraction of inspired oxygen [FiO 2]) to maintain saturation >92% (or >88% in known chronic obstructive pulmonary disease)
Heart rate: >130 beats per minute
Systolic blood pressure ≤90 mmHg or more than 40 mmHg below normal
Not passed urine in previous 18 hours, or for catheterised patients passed <0.5 mL/kg of urine per hour
Mottled or ashen appearance
Cyanosis of skin, lips, or tongue
Non-blanching petechial or purpuric rash of skin
Is at risk of neutropenic sepsis and presents with symptoms and signs of infection
See Febrile neutropenia.[139]
Carefully consider whether emergency medical care is required or whether the patient can be safely managed in the community with safety netting advice.[3] See box below on safety netting advice.
If you have decided to refer the patient for emergency medical care and have called for an emergency ambulance, you should start oxygen therapy in line with the following recommendations while awaiting the ambulance, if resources are available to do so.[50] Pulse oximetry should be available in all locations where emergency oxygen is used.[50]
Give oxygen immediately; an upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia.
Evidence: Target oxygen saturation in acutely ill adults
Too much supplemental oxygen increases mortality.
Evidence from a large systematic review and meta-analysis supports conservative/controlled oxygen therapy versus liberal oxygen therapy in acutely ill adults who are not at risk of hypercapnia.
Guidelines differ in their recommendations on target oxygen saturation in acutely unwell adults who are receiving supplemental oxygen.
The 2017 British Thoracic Society (BTS) guideline recommends a target SpO2 range of 94% to 98% for patients not at risk of hypercapnia, whereas the 2022 Thoracic Society of Australia and New Zealand (TSANZ) guideline recommends 92% to 96%.[50][164]
The 2022 Global Initiative For Asthma (GINA) guidelines recommend a target SpO2 range of 93% to 96% in the context of acute asthma exacerbations.[165]
One systematic review including a meta-analysis of data from 25 randomised controlled trials, published in 2018, found that in adults with acute illness, liberal oxygen therapy (broadly equivalent to a target saturation >96%) is associated with higher mortality than conservative oxygen therapy (broadly equivalent to a target saturation ≤96%).[138] In-hospital mortality was 11 per 1000 higher for the liberal oxygen therapy group versus the conservative therapy group (95% CI, 2-22 per 1000 more). Mortality at 30 days was also higher in the group who had received liberal oxygen (relative risk 1.14, 95% CI 1.01 to 1.29). The trials included adults with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery. Studies that were limited to people with chronic respiratory illness or psychiatric illness, patients on extracorporeal life support, those receiving hyperbaric oxygen therapy, or patients having elective surgery, were all excluded from the review.
An upper SpO2 limit of 96% is therefore reasonable when administering supplemental oxygen to patients with acute illness who are not at risk of hypercapnia. However, a higher target may be appropriate for some specific conditions (e.g., pneumothorax, carbon monoxide poisoning, cluster headache, and sickle cell crisis).[166]
In 2019 the BTS reviewed its guidance in response to this systematic review and meta-analysis and decided an interim update was not required.[167]
The committee noted that the systematic review supported the use of controlled oxygen therapy to a target.
While the systematic review showed an association between higher oxygen saturations and higher mortality, the BTS committee felt the review was not definitive on what the optimal target range should be. The suggested range of 94% to 96% in the review was based on the lower 95% confidence interval and the median baseline SpO2 from the liberal oxygen groups, along with the earlier 2015 TSANZ guideline recommendation.
Subsequently, experience during the COVID-19 pandemic has also made clinicians more aware of the feasibility of permissive hypoxaemia.[168] The BTS guidance is due for a review in 2022.
Management of oxygen therapy in patients in intensive care is specialised and informed by further evidence (not covered in this summary) that is more specific to this setting.[169][170][171]
Ensure you have a mechanism in place to administer antibiotics to any high-risk patient (either at your practice or via the ambulance service) if the transfer time to hospital is likely to be more than 1 hour.[3]
Ambulance crews should evaluate the risk of severe illness or death from sepsis using NEWS2 and consider a time-critical transfer and pre-alerting the hospital for patients with suspected or confirmed infection who either have consecutive NEWS2 scores of 5 or above or show cause for significant clinical concern.[3]
Paramedics who are thinking about giving antibiotics should follow local guidelines or seek advice from more senior colleagues, if needed.[3]
For patients at high risk of severe illness or death from sepsis who are in an acute mental health setting, follow local emergency protocols on treatment and ambulance transfer.[3]
Practical tip
If you need to refer a patient for emergency medical care in hospital, it is important to inform the hospital clinical team that the patient is on the way. This will enable the hospital to initiate appropriate treatment as soon as the patient arrives.
Management in the community
In a patient with signs and symptoms of an infection and evidence of physiological deterioration, presume sepsis until it can safely be excluded. Take a cautious approach when deciding whether it is safe to treat the patient’s infection in the community. Using your clinical judgement in making a decision is paramount. In particular, carefully consider the need for hospital admission if:[51][73]
The patient has one or more NICE high-risk criteria for sepsis
The patient appears seriously unwell to you, based on experience and clinical judgement
The patient lives alone with poor access to communication and/or transport
A carer or parent expresses serious concern about the patient (e.g., “they’re just not right”).
For details of the NICE risk criteria, see Diagnosis recommendations.
Treat the patient’s infection in line with local protocols and accepted practice. Antimicrobial prescribing guidelines from Public Health England and NICE are available for general practitioners in the UK.[200][201]
For patients at high risk of severe illness or death from sepsis who are in an acute mental health setting, follow local emergency protocols on treatment and ambulance transfer.[3]
Practical tip
If you decide that the patient is safe to treat in the community, written and verbal safety netting is vital.[51] Ensure the information is clear and specific rather than generalised advice; for example, do not say to “come back if you get worse” – instead specify key symptoms to watch out for (such as a non-blanching rash, change in behaviour or mental state, mottled skin, or ashen appearance) and explain where and how to access immediate medical care both in and out of hours.[51]
If you give the patient any safety netting advice, ensure you document this clearly in their medical notes, along with the patient’s observations and whether you have offered them any antibiotics. The 2015 national confidential enquiry into sepsis deaths found recorded evidence that safety netting advice had been provided in fewer than one quarter of cases.[61]
The UK Sepsis Trust advises the following acronym:[47]
Slurred speech or confusion
Extreme shivering or muscle pain
Passing no urine (in a day)
Severe breathlessness
‘ I feel I might die’
Skin mottled, ashen, blue, or very pale.
Advise the patient to call the emergency services if any of these symptoms develop. If the patient has a change in condition or deterioration that is not covered by the acronym above, advise them to arrange another appointment to see their general practitioner or to call their out of hours service provider.
It is also good practice to consider arranging a next-day review appointment or telephone call; if you will be unable to review the patient yourself, provide a written handover for your colleagues.
Use of this content is subject to our disclaimer
