Brugada syndrome is usually diagnosed through a combination of clinical features, risk factors, and ECG findings, although the diagnosis is considered probable or definite if spontaneous type 1 Brugada pattern is recorded on ECG from the 2nd to 4th intercostal spaces.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[4]Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45(5):433-42.
http://www.ncbi.nlm.nih.gov/pubmed/22920782?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
Refer all patients with type 1 Brugada pattern (spontaneous or induced) to a cardiologist or cardiac electrophysiologist for further investigation. Key differentials, such as acute coronary syndrome, should be excluded.
The proposed Shanghai score may also be used to aid diagnosis.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
See Criteria.
[Figure caption and citation for the preceding image starts]: Diagnosis summary for Brugada syndrome [IMAGE KEY: EP = Electrophysiology; ICD = Implantable cardioverter defibrillator; ICS = Intercostal space; MRI = Magnetic resonance imaging; SCB = Sodium-channel blocker]Krahn AD, et al. Brugada syndrome. JACC Clin Electrophysiol 2022 Mar;8(3):386-405; used with permission [Citation ends].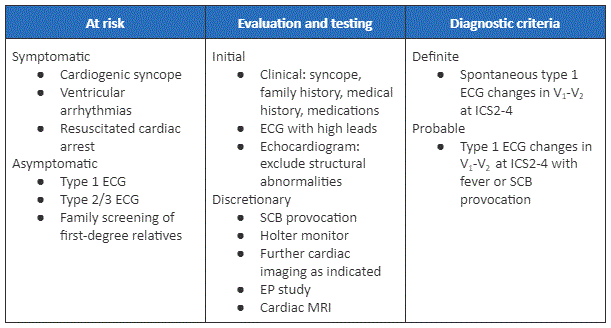
History
Take a detailed history of any patient with suspected BrS to identify clinical features of BrS, risk factors for BrS and sudden cardiac death, and other potential differentials. This is particularly important for the diagnosis of BrS in patients with type 1 Brugada pattern on ECG that is induced by triggers (e.g., fever, drugs such as sodium-channel blockers or psychotropics, alcohol, or illicit drugs), and in asymptomatic patients with type 2 or 3 Brugada pattern on ECG.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Clinical features
Consider a diagnosis of symptomatic BrS if a patient has a past history of any one or more of the following:
Unexplained cardiac arrest, or documented polymorphic ventricular tachycardia (PMVT), or ventricular fibrillation (VF)[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
There are many causes of sudden cardiac arrest, PMVT and VF, but BrS should be considered as a differential, particularly if there is Brugada pattern on ECG and one or more risk factors for BrS (see below).
Patients may also present with monomorphic VT, but this is rare and should prompt investigation of other arrhythmogenic cardiomyopathies.[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
[52]Rodríguez-Mañero M, Sacher F, de Asmundis C, et al. Monomorphic ventricular tachycardia in patients with Brugada syndrome: A multicenter retrospective study. Heart Rhythm. 2016;13(3):669-82.
http://www.ncbi.nlm.nih.gov/pubmed/26538325?tool=bestpractice.com
[53]Eckhardt LL. Monomorphic ventricular tachycardia in Brugada syndrome: True-true but related?. Heart Rhythm. 2016;13(3):683-5.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5404500
http://www.ncbi.nlm.nih.gov/pubmed/26632641?tool=bestpractice.com
Cardiogenic syncope
Up to one third of patients with BrS present with, or have a history of, syncope at the time of diagnosis.[54]Casado-Arroyo R, Berne P, Rao JY, Rodriguez-Mañero M, et al. Long-term trends in newly diagnosed Brugada syndrome: Implications for risk stratification. J Am Coll Cardiol. 2016 Aug 9;68(6):614-23.
https://www.sciencedirect.com/science/article/pii/S0735109716335458?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/27491905?tool=bestpractice.com
Take a careful history to differentiate cardiogenic from non-cardiogenic causes of syncope.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
The Shanghai score for the diagnosis of BrS assigns 2 points to patients with suspected cardiogenic syncope, and 1 point to those with an unclear cause of syncope.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[49]Antzelevitch C, Yan GX, Ackerman MJ, et al. J-Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. Heart Rhythm. 2016 Oct;13(10):e295-324.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5035208
http://www.ncbi.nlm.nih.gov/pubmed/27423412?tool=bestpractice.com
Patients with BrS who have a history of cardiogenic syncope are also at 2.5 to 5-fold greater risk of serious arrhythmic events compared with those with non-cardiogenic syncope.[47]Probst V, Veltmann C, Eckardt L, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121(5):635-43.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.109.887026?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/20100972?tool=bestpractice.com
[48]Delise P, Allocca G, Marras E, et al. Risk stratification in individuals with the Brugada type 1 ECG pattern without previous cardiac arrest: usefulness of a combined clinical and electrophysiologic approach. Eur Heart J. 2011;32(2):169-76.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3021386
http://www.ncbi.nlm.nih.gov/pubmed/20978016?tool=bestpractice.com
[55]Okamura H, Kamakura T, Morita H, et al. Risk stratification in patients with Brugada syndrome without previous cardiac arrest – prognostic value of combined risk factors. Circ J. 2015;79(2):310-17.
https://www.jstage.jst.go.jp/article/circj/79/2/79_CJ-14-1059/_html/-char/en
http://www.ncbi.nlm.nih.gov/pubmed/25428522?tool=bestpractice.com
[56]Kawazoe H, Nakano Y, Ochi H, et al. Risk stratification of ventricular fibrillation in Brugada syndrome using noninvasive scoring methods. Heart Rhythm. 2016 Oct;13(10):1947-54.
http://www.ncbi.nlm.nih.gov/pubmed/27424075?tool=bestpractice.com
[57]Honarbakhsh S, Providencia R, Garcia-Hernandez J, et al. A Primary prevention clinical risk score model for patients with Brugada syndrome (BRUGADA-RISK). JACC Clin Electrophysiol. 2021 Feb;7(2):210-2.
https://www.sciencedirect.com/science/article/pii/S2405500X20308197?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/33602402?tool=bestpractice.com
Patients with non-cardiogenic syncope have not been shown to have an increased risk for serious arrhythmic events.[58]Olde Nordkamp LR, Vink AS, Wilde AA, et al. Syncope in Brugada syndrome: prevalence, clinical significance, and clues from history taking to distinguish arrhythmic from nonarrhythmic causes. Heart Rhythm. 2015;12(2):367-75.
http://www.ncbi.nlm.nih.gov/pubmed/25311410?tool=bestpractice.com
Other clinical features of BrS may include:
Atrial fibrillation or flutter in a patient <30 years.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[59]Schimpf R, Giustetto C, Eckardt L, et al. Prevalence of supraventricular tachyarrhythmias in a cohort of 115 patients with Brugada syndrome. Ann Noninvasive Electrocardiol. 2008;13(3):266-9
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6932135
http://www.ncbi.nlm.nih.gov/pubmed/18713327?tool=bestpractice.com
[60]Kusano KF, Taniyama M, Nakamura K, et al. Atrial fibrillation in patients with Brugada syndrome relationships of gene mutation, electrophysiology, and clinical backgrounds. J Am Coll Cardiol. 2008;51(12):1169-75.
https://www.sciencedirect.com/science/article/pii/S0735109708000648?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/18355654?tool=bestpractice.com
Concomitant atrial arrhythmias are common in patients with BrS, with a prevalence of approximately 10%, although these are not specific for diagnosis of BrS.[61]Giustetto C, Cerrato N, Gribaudo E, et al. Atrial fibrillation in a large population with Brugada electrocardiographic pattern: prevalence, management, and correlation with prognosis. Heart Rhythm. 2014;11(2):259-65.
http://www.ncbi.nlm.nih.gov/pubmed/24513919?tool=bestpractice.com
Use of sodium-channel blockers may unmask the Brugada pattern in some of these patients.[61]Giustetto C, Cerrato N, Gribaudo E, et al. Atrial fibrillation in a large population with Brugada electrocardiographic pattern: prevalence, management, and correlation with prognosis. Heart Rhythm. 2014;11(2):259-65.
http://www.ncbi.nlm.nih.gov/pubmed/24513919?tool=bestpractice.com
Nocturnal agonal respirations.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
The Shanghai score for BrS assigns 2 points to patients with nocturnal agonal respirations.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[49]Antzelevitch C, Yan GX, Ackerman MJ, et al. J-Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. Heart Rhythm. 2016 Oct;13(10):e295-324.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5035208
http://www.ncbi.nlm.nih.gov/pubmed/27423412?tool=bestpractice.com
In a Danish national survey, prevalence of nocturnal agonal respirations in patients with BrS was 14%, but this is likely to be greater in endemic areas.[62]Holst AG, Jensen HK, Eschen O, et al. Low disease prevalence and inappropriate implantable cardioverter defibrillator shock rate in Brugada syndrome: a nationwide study. Europace. 2012;14(7):1025-9.
https://core.ac.uk/reader/50680417?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/22286273?tool=bestpractice.com
Nocturnal agonal respirations were described in early reports from Southeast Asia (Philippines, Thailand, Japan, China, and others) of sudden unexpected nocturnal death syndrome (SUNDS), a syndrome which is thought to be genetically, phenotypically, and functionally the same as BrS.[63]Vatta M, Dumaine R, Antzelevitch C, et al. Novel mutations in domain I of SCN5A cause Brugada syndrome. Mol Genet Metab. 2002;75(4):317-24.
http://www.ncbi.nlm.nih.gov/pubmed/12051963?tool=bestpractice.com
Risk factors
Identify any of the following risk factors which are associated with, or increase the risk of, BrS and subsequent sudden cardiac death:[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Age 30 to 50 years[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
BrS is typically first diagnosed in young to middle-aged patients.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Patients aged 30 to 50 years with BrS are also at high risk of serious arrhythmic events.[39]Milman A, Andorin A, Gourraud JB, et al. Age of first arrhythmic event in Brugada syndrome: Data from the SABRUS (Survey on Arrhythmic Events in Brugada Syndrome) in 678 patients. Circ Arrhythm Electrophysiol. 2017 Dec;10(12):e005222.
https://iris.unito.it/handle/2318/1660808
http://www.ncbi.nlm.nih.gov/pubmed/29254945?tool=bestpractice.com
This is also typically the age at which patients with BrS may present with their first serious arrhythmic event.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
However, serious arrhythmic events have also been reported in children as young as <1 year old.[20]Rattanawong P, Vutthikraivit W, Charoensri A, et al. Fever-induced Brugada syndrome is more common than previously suspected: A cross-sectional study from an endemic area. Ann Noninvasive Electrocardiol. 2016;21(2):136-41.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6931454
http://www.ncbi.nlm.nih.gov/pubmed/26178440?tool=bestpractice.com
[40]Gonzalez Corcia MC, Sieira J, Sarkozy A, et al. Brugada syndrome in the young: an assessment of risk factors predicting future events. Europace. 2017 Nov 1;19(11):1864-73.
https://academic.oup.com/europace/article/19/11/1864/2194460?login=false
http://www.ncbi.nlm.nih.gov/pubmed/27738063?tool=bestpractice.com
[41]Minier M, Probst V, Berthome P, et al. Age at diagnosis of Brugada syndrome: Influence on clinical characteristics and risk of arrhythmia. Heart Rhythm. 2020 May;17(5 Pt A):743-9.
http://www.ncbi.nlm.nih.gov/pubmed/31790831?tool=bestpractice.com
Women in particular may be more likely to have their first SAE in childhood, or later in life.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[39]Milman A, Andorin A, Gourraud JB, et al. Age of first arrhythmic event in Brugada syndrome: Data from the SABRUS (Survey on Arrhythmic Events in Brugada Syndrome) in 678 patients. Circ Arrhythm Electrophysiol. 2017 Dec;10(12):e005222.
https://iris.unito.it/handle/2318/1660808
http://www.ncbi.nlm.nih.gov/pubmed/29254945?tool=bestpractice.com
Patients >50 years who have BrS experience significantly fewer serious arrhythmic events compared with younger patients, and for those ≥55 years at the time of BrS diagnosis, annual mortality is similar to that of the general population.[41]Minier M, Probst V, Berthome P, et al. Age at diagnosis of Brugada syndrome: Influence on clinical characteristics and risk of arrhythmia. Heart Rhythm. 2020 May;17(5 Pt A):743-9.
http://www.ncbi.nlm.nih.gov/pubmed/31790831?tool=bestpractice.com
[42]Juang JM, Chen CY, Chen YH, et al. Prevalence and prognosis of Brugada electrocardiogram patterns in an elderly Han Chinese population: a nation-wide community-based study (HALST cohort). Europace. 2015 Oct;17(Suppl 2):ii54-62.
https://academic.oup.com/europace/article/17/suppl_2/ii54/2802554?login=false
http://www.ncbi.nlm.nih.gov/pubmed/26842116?tool=bestpractice.com
[43]Kitamura T, Fukamizu S, Kawamura I, et al. Clinical characteristics and long-term prognosis of senior patients with Brugada syndrome. JACC Clin Electrophysiol. 2017 Jan;3(1):57-67.
https://www.sciencedirect.com/science/article/pii/S2405500X16301062?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/29759696?tool=bestpractice.com
However, it remains unclear whether these observations are due to protective effects acquired through ageing or simply represent selection bias.
Male sex[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
BrS is more common in men compared with women.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[39]Milman A, Andorin A, Gourraud JB, et al. Age of first arrhythmic event in Brugada syndrome: Data from the SABRUS (Survey on Arrhythmic Events in Brugada Syndrome) in 678 patients. Circ Arrhythm Electrophysiol. 2017 Dec;10(12):e005222.
https://iris.unito.it/handle/2318/1660808
http://www.ncbi.nlm.nih.gov/pubmed/29254945?tool=bestpractice.com
Men account for up to 90% of cases of BrS in some cohorts.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[39]Milman A, Andorin A, Gourraud JB, et al. Age of first arrhythmic event in Brugada syndrome: Data from the SABRUS (Survey on Arrhythmic Events in Brugada Syndrome) in 678 patients. Circ Arrhythm Electrophysiol. 2017 Dec;10(12):e005222.
https://iris.unito.it/handle/2318/1660808
http://www.ncbi.nlm.nih.gov/pubmed/29254945?tool=bestpractice.com
Evidence has shown that men with BrS are also at increased risk of serious arrhythmic events.[44]Yuan M, Tian C, Li X, et al. Gender differences in prognosis and risk stratification of Brugada syndrome: A pooled analysis of 4,140 patients from 24 clinical trials. Front Physiol. 2018 Aug 22;9:1127.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6113678
http://www.ncbi.nlm.nih.gov/pubmed/30246798?tool=bestpractice.com
[45]Sieira J, Brugada P. Brugada syndrome: Defining the risk in asymptomatic patients. Arrhythm Electrophysiol Rev. 2016;5(3):164-9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5248661
http://www.ncbi.nlm.nih.gov/pubmed/28116080?tool=bestpractice.com
[46]Berthome P, Tixier R, Briand J, et al. Clinical presentation and follow-up of women affected by Brugada syndrome. Heart Rhythm. 2019 Feb;16(2):260-7.
http://www.ncbi.nlm.nih.gov/pubmed/30193851?tool=bestpractice.com
However, this finding remains controversial, and has not been confirmed with complete adjustment for potential confounding factors.[47]Probst V, Veltmann C, Eckardt L, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121(5):635-43.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.109.887026?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/20100972?tool=bestpractice.com
[48]Delise P, Allocca G, Marras E, et al. Risk stratification in individuals with the Brugada type 1 ECG pattern without previous cardiac arrest: usefulness of a combined clinical and electrophysiologic approach. Eur Heart J. 2011;32(2):169-76.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3021386
http://www.ncbi.nlm.nih.gov/pubmed/20978016?tool=bestpractice.com
Asian ancestry[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
BrS occurs more commonly in Asia compared with Europe and the US.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[13]Mizusawa Y, Wilde AA. Brugada syndrome. Circ Arrhythm Electrophysiol. 2012;5(3):606-16.
https://www.ahajournals.org/doi/10.1161/CIRCEP.111.964577?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/22715240?tool=bestpractice.com
BrS is particularly common in Southeast Asia, where a nocturnal sudden death syndrome in young men was clinically reported by a variety of local names in countries such as Philippines, Thailand, Japan, and China, prior to the formal classification of BrS.[14]Sarquella-Brugada G, Campuzano O, Arbelo E, Brugada J, Brugada R. Brugada syndrome: clinical and genetic findings. Genet Med. 2016;18(1):3-12.
https://linkinghub.elsevier.com/retrieve/pii/S1098-3600(21)04299-4
http://www.ncbi.nlm.nih.gov/pubmed/25905440?tool=bestpractice.com
Positive family history
Determine the following features (which form part of the Shanghai score) in any first or second degree relative of the patient:[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[49]Antzelevitch C, Yan GX, Ackerman MJ, et al. J-Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. Heart Rhythm. 2016 Oct;13(10):e295-324.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5035208
http://www.ncbi.nlm.nih.gov/pubmed/27423412?tool=bestpractice.com
Definite BrS
Suspicious sudden cardiac death (febrile, nocturnal, confounded by BrS-associated drug)
Unexplained sudden cardiac death <45 years with non-contributory autopsy.
Bear in mind that, although family history is a standard and important part of any diagnostic evaluation, limited evidence exists to support a family history of sudden death as a risk factor for BrS-associated arrhythmic events.[50]Kamakura S, Ohe T, Nakazawa K, et al. Long-term prognosis of probands with Brugada-pattern ST-elevation in leads V1-V3. Circ Arrhythm Electrophysiol. 2009;2(5):495-503.
https://www.ahajournals.org/doi/10.1161/CIRCEP.108.816892?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/19843917?tool=bestpractice.com
Asymptomatic patients
Features of BrS may only be apparent when induced by certain factors.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Inducible features are particularly important to identify in asymptomatic patients (i.e., those who do not have a past history of unexplained cardiac arrest, ventricular arrhythmias, or syncope), but may also be present in symptomatic patients. These include:[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Febrile illness
Known to induce type 1 Brugada pattern on ECG and precipitate arrhythmic events.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
In endemic regions, the prevalence of BrS in patients presenting with febrile illness for any reason has been reportedly as high as 4%, around 20 times greater than a non-febrile control group in one Thai cross-sectional study of 401 patients.[19]Adler A, Topaz G, Heller K, et al. Fever-induced Brugada pattern: how common is it and what does it mean?. Heart Rhythm. 2013;10(9):1375-82.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3832740
http://www.ncbi.nlm.nih.gov/pubmed/23872691?tool=bestpractice.com
[20]Rattanawong P, Vutthikraivit W, Charoensri A, et al. Fever-induced Brugada syndrome is more common than previously suspected: A cross-sectional study from an endemic area. Ann Noninvasive Electrocardiol. 2016;21(2):136-41.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6931454
http://www.ncbi.nlm.nih.gov/pubmed/26178440?tool=bestpractice.com
While in isolation fever is not specific for BrS, in a patient with suspected BrS, it has implications for both diagnosis and management.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Drugs
These include sodium-channel blockers (e.g., flecainide, procainamide), psychotropics (e.g., tricyclic or tetracyclic antidepressants, lithium), and local anaesthetics.
Known to induce type 1 Brugada ECG changes, and may also precipitate arrhythmic events.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[17]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
Illicit drugs or alcohol
Known to induce type 1 Brugada ECG changes and may also precipitate arrhythmic events.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[17]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
Physical exam
Perform a full cardiovascular examination to identify other causes of the patient’s clinical presentation. These include:
Acute coronary syndrome
Arrhythmogenic cardiomyopathy
Athlete’s heart
Haemodynamically significant valvular disease
Pectus excavatum
Incomplete right bundle branch block.
Although the physical exam is valuable in the overall evaluation of any patient, it is not useful to rule out or rule in a diagnosis of BrS.
Check whether the patient has a fever, which may unmask features of BrS.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Initial investigations
ECG
Perform an ECG as the first line investigation for any patient with suspected BrS.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[17]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[64]Nielsen JC, Lin YJ, de Oliveira Figueiredo MJ, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: use the right tool for the right outcome, in the right population. J Arrhythm. 2020 Jun 15;36(4):553-607.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/32782627
http://www.ncbi.nlm.nih.gov/pubmed/32782627?tool=bestpractice.com
Look for the following patterns that may indicate BrS:[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Type 1: coved ST-segment elevation (J-point elevation with a gradual down-sloping ST-segment) ≥2 mm with a negative T-wave in the right precordial leads.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[4]Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45(5):433-42.
http://www.ncbi.nlm.nih.gov/pubmed/22920782?tool=bestpractice.com
[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
Type 1 Brugada pattern may be spontaneous, or induced (e.g., by factors such as fever or sodium-channel blockers).[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Type 2 or 3: saddleback ST-segment configuration with variable levels of ST-segment elevation.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[4]Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45(5):433-42.
http://www.ncbi.nlm.nih.gov/pubmed/22920782?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
Consider high precordial lead testing if ECG using standard lead placement is not conclusive and you have a high clinical suspicion of BrS. High precordial lead testing increases diagnostic yield by accounting for anatomical variations in right ventricular outflow tract anatomy.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[4]Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45(5):433-42.
http://www.ncbi.nlm.nih.gov/pubmed/22920782?tool=bestpractice.com
[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
[65]Veltmann C, Papavassiliu T, Konrad T, et al. Insights into the location of type I ECG in patients with Brugada syndrome: correlation of ECG and cardiovascular magnetic resonance imaging. Heart Rhythm. 2012;9(3):414-21.
http://www.ncbi.nlm.nih.gov/pubmed/22119454?tool=bestpractice.com
[66]Sangwatanaroj S, Prechawat S, Sunsaneewitayakul B, et al. New electrocardiographic leads and the procainamide test for the detection of the Brugada sign in sudden unexplained death syndrome survivors and their relatives. Eur Heart J. 2001;22(24):2290-6.
https://academic.oup.com/eurheartj/article/22/24/2290/512888?login=true
http://www.ncbi.nlm.nih.gov/pubmed/11728150?tool=bestpractice.com
[67]Govindan M, Batchvarov VN, Raju H, et al. Utility of high and standard right precordial leads during ajmaline testing for the diagnosis of Brugada syndrome. Heart. 2010;96(23):1904-8.
http://www.ncbi.nlm.nih.gov/pubmed/20962343?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Electrocardiographic patterns in Brugada syndrome showing type 1 (diagnostic) and types 2 and 3 (non-diagnostic) patterns. Type-1 (diagnostic): coved STT morphology in lead V2 with J-point elevation (dark grey line) of ≥0.2 mV (≥2 mm) and a terminal ST-segment elevation (light grey line, J+60 ms) also ≥0.2 mV (≥2 mm). Note the PR interval and wider QRS complex, wide and deep S in lead I, and fractionation in the right precordial ECG leads. Type-2 (non-diagnostic): saddleback STT morphology in lead V2 with J-point elevation (dark grey line) of ≥0.2 mV (≥2 mm) and a terminal ST-segment elevation (light grey line, J+60 ms) ≥0.1 mV (≥1 mm), followed by a positive T wave. Note the less wide and deep S-wave in lead I, less prominent fractionation. Type-3 (non-diagnostic): saddleback STT morphology in lead V2 with J-point elevation (dark grey line) of ≥0.2 mV (≥2 mm) and a terminal ST-segment elevation (light grey line, J+60 ms) <0.1 mV (<1 mm)Marsman EMJ et al. Heart 2022 May;108(9):668-75; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Standard- and high-lead ECG positions. (Top) Standard-lead ECG positions and corresponding precordial ECG in a patient with Brugada syndrome. (Bottom) High-lead ECG positions and corresponding ECG in the same patient. Note that hV5 and hV6 on the high-lead ECG correspond with V1 and V2 on the standard-lead ECG.Krahn AD, et al. Brugada syndrome. JACC Clin Electrophysiol 2022 Mar;8(3):386-405; used with permission [Citation ends].
Refer all patients with type 1 Brugada pattern (spontaneous or induced) to a cardiologist or cardiac electrophysiologist.
The diagnosis of BrS is considered probable or definite if spontaneous type 1 Brugada pattern is recorded on ECG from the 2nd to 4th intercostal spaces.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[4]Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45(5):433-42.
http://www.ncbi.nlm.nih.gov/pubmed/22920782?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
However, these patients require diagnostic evaluation by a specialist.
Ensure you establish the patient’s clinical history in all patients with type 1 Brugada pattern.[4]Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45(5):433-42.
http://www.ncbi.nlm.nih.gov/pubmed/22920782?tool=bestpractice.com
This is essential, because distinguishing type 1 Brugada pattern from other causes of ST elevation using ECG alone can be difficult, even for an experienced cardiologist.[4]Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45(5):433-42.
http://www.ncbi.nlm.nih.gov/pubmed/22920782?tool=bestpractice.com
If the patient has induced type 1 Brugada pattern, additional information is key to aid diagnosis of BrS (e.g., relevant clinical history or family history, and/or genetic testing).[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
If the patient has type 2 or 3 Brugada pattern, consider provocative drug testing with sodium channel blockade (see below).[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[17]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
Echocardiogram
Organise an echocardiogram for any patient being evaluated for BrS to assess for underlying structural heart disease and to rule out other causes of their presentation.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[49]Antzelevitch C, Yan GX, Ackerman MJ, et al. J-Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. Heart Rhythm. 2016 Oct;13(10):e295-324.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5035208
http://www.ncbi.nlm.nih.gov/pubmed/27423412?tool=bestpractice.com
Echocardiogram is often normal in BrS or may demonstrate mild structural abnormalities in the right ventricle or right ventricular outflow tract.
Other investigations
Provocative drug testing with sodium channel blockade
Consider provocative drug testing with sodium-channel blockers (e.g., procainamide, flecainide) for any patient with both:[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[13]Mizusawa Y, Wilde AA. Brugada syndrome. Circ Arrhythm Electrophysiol. 2012;5(3):606-16.
https://www.ahajournals.org/doi/10.1161/CIRCEP.111.964577?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/22715240?tool=bestpractice.com
[17]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[49]Antzelevitch C, Yan GX, Ackerman MJ, et al. J-Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. Heart Rhythm. 2016 Oct;13(10):e295-324.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5035208
http://www.ncbi.nlm.nih.gov/pubmed/27423412?tool=bestpractice.com
Type 2 or 3 Brugada pattern on ECG
AND
Suspected BrS due to relevant clinical signs, symptoms, or family history.
The diagnosis of BrS is considered probable in these patients if type 1 Brugada ECG pattern is provoked during drug testing with sodium-channel blockers; this scores two points on the Shanghai score.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Refer these patients to a cardiologist or cardiac electrophysiologist.
Due to an associated risk of inducing life-threatening ventricular arrhythmias, provocative drug testing should only be performed by experts under optimal circumstances.[68]Arnar DO, Mairesse GH, Boriani G, et al. Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS). Europace. 2019 Mar 18;21(6):844–5.
https://academic.oup.com/europace/article/21/6/844/5382236?login=false
http://www.ncbi.nlm.nih.gov/pubmed/30882141?tool=bestpractice.com
Sodium-channel blocker test is not recommended in patients with a prior type I Brugada pattern.[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Genetic testing
Arrange genetic testing in patients with type 1 Brugada pattern on ECG (spontaneous or induced); this helps facilitate family screening of first degree relatives.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[17]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[69]Gollob MH, Blier L, Brugada R, et al. Recommendations for the use of genetic testing in the clinical evaluation of inherited cardiac arrhythmias associated with sudden cardiac death: Canadian Cardiovascular Society/Canadian Heart Rhythm Society joint position paper. Can J Cardiol. 2011 Mar-Apr;27(2):232-45.
https://www.onlinecjc.ca/article/S0828-282X(10)00094-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21459272?tool=bestpractice.com
However, the presence of a susceptible gene mutation is not diagnostic of BrS. This is in part due to only 50% penetrance of genetic variants. Clinical presentation remains central to diagnosing Brugada syndrome.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Probable pathogenic mutation in a BrS susceptibility gene also scores 0.5 points on the Shanghai score.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Mutations of many genes have been implicated in BrS, but only SCN5A gene variants are considered definitely disease-causing.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
However, identifiable SCN5A variants are only found in approximately 20% to 30% of patients with BrS.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[15]Kapplinger JD, Tester DJ, Alders M, et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010 Jan;7(1):33-46.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2822446
http://www.ncbi.nlm.nih.gov/pubmed/20129283?tool=bestpractice.com
[16]Hu D, Barajas-Martínez H, Pfeiffer R, et al. Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J Am Coll Cardiol. 2014;64(1):66-79.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4116276
http://www.ncbi.nlm.nih.gov/pubmed/24998131?tool=bestpractice.com
[17]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[64]Nielsen JC, Lin YJ, de Oliveira Figueiredo MJ, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: use the right tool for the right outcome, in the right population. J Arrhythm. 2020 Jun 15;36(4):553-607.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/32782627
http://www.ncbi.nlm.nih.gov/pubmed/32782627?tool=bestpractice.com
Mutations of other genes only account for around 2% to 5% of cases.[14]Sarquella-Brugada G, Campuzano O, Arbelo E, Brugada J, Brugada R. Brugada syndrome: clinical and genetic findings. Genet Med. 2016;18(1):3-12.
https://linkinghub.elsevier.com/retrieve/pii/S1098-3600(21)04299-4
http://www.ncbi.nlm.nih.gov/pubmed/25905440?tool=bestpractice.com
[15]Kapplinger JD, Tester DJ, Alders M, et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010 Jan;7(1):33-46.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2822446
http://www.ncbi.nlm.nih.gov/pubmed/20129283?tool=bestpractice.com
[16]Hu D, Barajas-Martínez H, Pfeiffer R, et al. Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J Am Coll Cardiol. 2014;64(1):66-79.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4116276
http://www.ncbi.nlm.nih.gov/pubmed/24998131?tool=bestpractice.com
[18]Hosseini SM, Kim R, Udupa S, et al. Reappraisal of reported genes for sudden arrhythmic death: Evidence-based evaluation of gene validity for Brugada syndrome. Circulation. 2018 Sep 18;138(12):1195-205.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6147087
http://www.ncbi.nlm.nih.gov/pubmed/29959160?tool=bestpractice.com
Advanced cardiac imaging (MRI or CT)
Consider other advanced imaging modalities, such as cardiac MRI or CT, if the diagnosis of BrS is uncertain to help differentiate BrS from other differentials, such as arrhythmogenic cardiomyopathy.[36]Bastiaenen R, Cox AT, Castelletti S, et al. Late gadolinium enhancement in Brugada syndrome: A marker for subtle underlying cardiomyopathy? Heart Rhythm. 2017 Apr;14(4):583-9.
http://www.ncbi.nlm.nih.gov/pubmed/27919765?tool=bestpractice.com
[70]Heermann P, Fritsch H, Koopmann M, et al. Biventricular myocardial strain analysis using cardiac magnetic resonance feature tracking (CMR-FT) in patients with distinct types of right ventricular diseases comparing arrhythmogenic right ventricular cardiomyopathy (ARVC), right ventricular outflow-tract tachycardia (RVOT-VT), and Brugada syndrome (BrS). Clin Res Cardiol. 2019 Oct;108(10):1147-62.
http://www.ncbi.nlm.nih.gov/pubmed/30868222?tool=bestpractice.com
See Differentials.
Invasive electrophysiological (EP) study with inducibility testing for ventricular arrhythmias
Invasive EP studies with inducibility testing (including measurement of baseline intervals, programmed electrical stimulation [PES], and electroanatomical mapping) may be considered following consultation with an electrophysiologist or cardiologist with expertise in managing BrS if the patient has asymptomatic BrS and is deemed intermediate risk on risk stratification. See Management approach for more information about risk stratification.[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[64]Nielsen JC, Lin YJ, de Oliveira Figueiredo MJ, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: use the right tool for the right outcome, in the right population. J Arrhythm. 2020 Jun 15;36(4):553-607.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/32782627
http://www.ncbi.nlm.nih.gov/pubmed/32782627?tool=bestpractice.com
Although there is debate regarding the prognostic value of PES in primary electrical diseases, there is some evidence to consider its use in BrS.[64]Nielsen JC, Lin YJ, de Oliveira Figueiredo MJ, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: use the right tool for the right outcome, in the right population. J Arrhythm. 2020 Jun 15;36(4):553-607.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/32782627
http://www.ncbi.nlm.nih.gov/pubmed/32782627?tool=bestpractice.com
[71]Borgquist R, Haugaa KH, Gilljam T, et al. The diagnostic performance of imaging methods in ARVC using the 2010 Task Force criteria. Eur Heart J Cardiovasc Imaging. 2014;15(11):1219-25.
https://academic.oup.com/ehjcimaging/article/15/11/1219/2399659?login=false
http://www.ncbi.nlm.nih.gov/pubmed/24939949?tool=bestpractice.com
Invasive EP assessments have demonstrated voltage abnormalities recorded from the right ventricular epicardium of patients with BrS in the absence of cardiac MR or CT structural abnormalities.[38]Nademanee K, Veerakul G, Chandanamattha P, et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123(12):1270-9.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.110.972612?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/21403098?tool=bestpractice.com
This highlights the importance of expert consultation if you suspect BrS, but the diagnosis is unclear.


