Investigations
1st investigations to order
FBC with differential
Test
Most patients with acute myeloid leukaemia (AML) or acute promyelocytic leukaemia (APL) have anaemia, neutropenia, and/or thrombocytopenia, but blood count can vary greatly.
An elevated white blood cell (WBC) count >100 × 10⁹/L (>100,000/microlitre; hyperleukocytosis) occurs in approximately 5% to 20% of patients with AML, predisposing to complications such as tumour lysis syndrome, central nervous system involvement, and leukostasis (symptomatic hyperleukocytosis; symptoms include respiratory distress and altered mental status).[3][4] These are medical emergencies and require immediate treatment. Despite the elevation in WBC count, many patients have severe neutropenia (absolute neutrophil count [ANC] <0.5 × 10⁹/L [<500/microlitre]), thus placing them at high risk for serious infections.
Result
anaemia, macrocytosis, leukocytosis, neutropenia, and/or thrombocytopenia
peripheral blood smear
Test
Blasts are immature cells and are not normally seen in the peripheral blood.
The acute myeloid leukaemia (AML) blood film may show myeloid blasts characterised by Auer rods or Phi bodies.
In acute promyelocytic leukaemia (APL), the blood film will typically show hypergranular promyelocytes with bilobed nuclei and bundles of Auer rods (as well as myeloid blasts).
A variant of APL is characterised by hypogranular promyelocytes (absence of Auer rods), but is less common.
[Figure caption and citation for the preceding image starts]: Peripheral blood film of a patient with acute promyelocytic leukaemia showing hypergranular promyelocytes, some with bundles of Auer rodsFrom the collection of Drs K. Raj and P. Mehta; used with patient consent [Citation ends].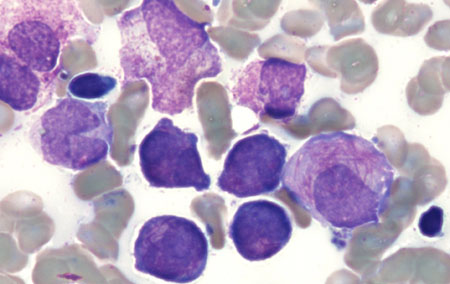 [Figure caption and citation for the preceding image starts]: Peripheral blood film of a patient with acute promyelocytic leukaemia showing hypergranular promyelocytes with bi-lobed nuclei and bundles of Auer rodsFrom the collection of Drs K. Raj and P. Mehta; used with patient consent [Citation ends].
[Figure caption and citation for the preceding image starts]: Peripheral blood film of a patient with acute promyelocytic leukaemia showing hypergranular promyelocytes with bi-lobed nuclei and bundles of Auer rodsFrom the collection of Drs K. Raj and P. Mehta; used with patient consent [Citation ends].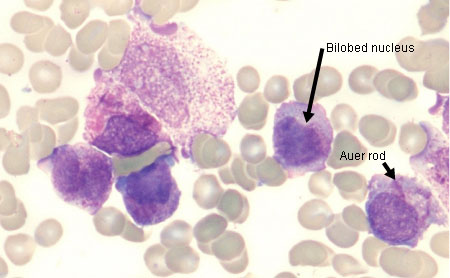 [Figure caption and citation for the preceding image starts]: Peripheral blood film of a patient with acute myeloid leukaemia with maturation showing myeloid blasts with an Auer rodFrom the collection of Drs K. Raj and P. Mehta; used with patient consent [Citation ends].
[Figure caption and citation for the preceding image starts]: Peripheral blood film of a patient with acute myeloid leukaemia with maturation showing myeloid blasts with an Auer rodFrom the collection of Drs K. Raj and P. Mehta; used with patient consent [Citation ends].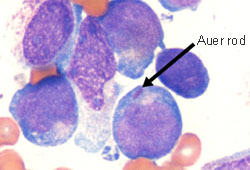
Result
myeloid blasts on blood film; presence of Auer rods or Phi bodies (in AML); presence of hypergranular promyelocytes with bilobed nuclei and bundles of Auer rods, or hypogranular promyelocytes without Auer rods (in APL)
coagulation panel
Test
Ordered as baseline and monitored throughout treatment.
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) may be mildly prolonged with normal fibrinogen and D-dimer.
If coagulation tests are abnormal (prolonged PT and aPTT, decreased fibrinogen, and/or raised D-dimer), disseminated intravascular coagulation (DIC) should be suspected and an urgent referral is warranted to commence treatment. Refer to the International Society on Thrombosis and Haemostasis (ISTH) scoring system for DIC.[61]
DIC occurs most frequently in acute promyelocytic leukaemia and is potentially life-threatening.[62]
Severely decreased fibrinogen suggests primary fibrinolysis.
Result
PT, aPTT, fibrinogen, and/or D-dimer may be normal or abnormal; if abnormal, DIC should be suspected
serum electrolytes
Test
Ordered as baseline and monitored throughout treatment.
Hyperkalaemia, hyperphosphataemia, and hypocalcaemia (together with hyperuricaemia and elevated serum lactate dehydrogenase) may occur due to tumour lysis syndrome (TLS), particularly during treatment and if white blood cell count (tumour burden) is high. This can lead to cardiac arrhythmias, seizures, acute kidney injury, and death, if untreated. TLS is an oncological emergency.[60] See Tumour lysis syndrome.
Hypercalcaemia may occur due to bony infiltration or ectopic release of a parathyroid hormone-like substance.
Result
serum potassium and phosphorus may be elevated; serum calcium may be decreased or elevated
serum uric acid
Test
Ordered as baseline and monitored throughout treatment.
Hyperuricaemia (together with hyperkalaemia, hyperphosphataemia, hypocalcaemia, and elevated serum lactate dehydrogenase) may occur due to tumour lysis syndrome (TLS), particularly during treatment and if white blood cell count (tumour burden) is high. This can lead to cardiac arrhythmias, seizures, acute kidney injury, and death, if untreated. TLS is an oncological emergency.[60] See Tumour lysis syndrome.
The degree of uric acid elevation may reflect the extent of disease burden and is useful for prognosis.[65]
Result
may be elevated
serum lactate dehydrogenase (LDH)
Test
Ordered as baseline and monitored throughout treatment.
Elevated serum LDH (together with hyperkalaemia, hyperphosphataemia, hyperuricaemia, and hypocalcaemia) may occur due to tumour lysis syndrome (TLS), particularly during treatment and if white blood cell count (tumour burden) is high. This can lead to cardiac arrhythmias, seizures, acute kidney injury, and death, if untreated. TLS is an oncological emergency.[60] See Tumour lysis syndrome.
The degree of LDH elevation may reflect the extent of disease burden and is useful for prognosis.[66]
Result
may be elevated
renal function
Test
Ordered as baseline and monitored throughout treatment.
Includes measurement of urea and creatinine.
Acute kidney injury may occur if tumour lysis syndrome (TLS) develops. TLS is an oncological emergency.[60] See Tumour lysis syndrome.
Result
may be abnormal if there is renal dysfunction
liver function tests
Test
Ordered as baseline and monitored throughout treatment.
Includes measurement of total bilirubin, albumin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST).
Result
may be abnormal if there is liver dysfunction
bone marrow evaluation
Test
Diagnosis requires a bone marrow aspirate and trephine biopsy analysis.[27][45]
Cytomorphology assessment demonstrates bone marrow hypercellularity and infiltration by myeloid blasts in AML (as well as hypergranular or hypogranular [less common] promyelocytes in acute promyelocytic leukaemia [APL]). Myeloid blast cells are negative for terminal deoxynucleotidyl transferase (TdT) and stain positive for myeloperoxidase.
Immunophenotyping using flow cytometry (on bone marrow aspirate) and immunohistochemistry (using core biopsy specimen) identifies cell surface and cytoplasmic markers of myeloid blasts (e.g., CD34, CD33) and establishes lineage.
Confirmation of a myeloid origin of the leukaemic cells by immunophenotyping is essential to differentiate acute myeloid leukaemia (AML) and acute lymphoblastic leukaemia (ALL), as these are often clinically indistinguishable.
If bone marrow specimens are inadequate or unattainable, then peripheral blood can be used for pathological assessment provided there are sufficient numbers of circulating blasts.[63]
Result
bone marrow hypercellularity and infiltration by myeloid blasts; presence of Auer rods or Phi bodies (in AML); presence of hypergranular promyelocytes with bilobed nuclei and bundles of Auer rods, or hypogranular promyelocytes without Auer rods (in APL); positive for cell-surface and cytoplasmic markers for myeloid blasts (e.g., CD34, CD33, myeloperoxidase); negative for TdT
genetic testing
Test
Cytogenetic analysis (karyotyping, fluorescence in situ hybridisation [FISH], or whole-genome sequencing) and molecular genetic testing (e.g., polymerase chain reaction [PCR], next-generation sequencing [NGS] assays) should be performed on bone marrow specimens (or peripheral blood if circulating blasts are present) to guide diagnosis, prognosis, risk stratification, and treatment.[27][45]
In acute myeloid leukaemia (AML), the following genetic abnormalities should be investigated due to their association with specific prognoses and treatment targets: RUNX1::RUNX1T1; CBFB::MYH11; MLLT3::KMT2A (or other KMT2A rearrangements); DEK::NUP214; BCR::ABL1; KAT6A::CREBBP; KIT; NPM1; FLT3 (ITD and TKD); IDH1; IDH2; CEBPA (basic leucine zipper [bZIP] domain); -5 or del(5q); -7; -17/abn(17p); GATA2; MECOM(EVI1); ASXL1; BCOR; EZH2; RUNX1; SF3B1; SRSF2; STAG2; U2AF1; ZRSR2; and TP53.[27][45] See Criteria.
Acute promyelocytic leukaemia (APL, a subtype of AML) is characterised by the PML::RARA fusion gene caused by a t(15;17)(q22;q12) balanced chromosomal rearrangement.[27][44]
Result
may identify AML-defining genetic abnormalities
Investigations to consider
genetic testing for heritable haematologic malignancy predisposition syndrome
Test
Should be considered for certain patients, such as those <50 years of age, and those with a family history of a heritable haematologic disorder.[27][45]
Findings may guide management.
Result
may identify a heritable haematologic malignancy predisposition syndrome (e.g., GATA2 deficiency syndrome, Shwachman-Diamond syndrome, dyskeratosis congenita)
human leukocyte antigen (HLA) typing
Test
Ordered to assess and match for suitable donor for allogeneic stem cell transplant.[45]
Result
variable
CNS imaging and lumbar puncture
Test
CNS imaging (e.g., brain MRI or CT scan) should be performed to detect CNS bleeding, meningeal disease, or mass lesions in patients who present with neurological signs or symptoms suggesting CNS involvement.[27][45]
If CNS imaging does not identify CNS bleeding or a mass effect, and neurological signs and symptoms persist, lumbar puncture is recommended.[45]
One dose of intrathecal chemotherapy (e.g., methotrexate or cytarabine, or a combination of both agents) can be considered at the time of diagnostic lumbar puncture.[45]
Coagulopathy should be managed before lumbar puncture, particularly in patients with acute promyelocytic leukaemia.
Result
CNS imaging may show intracranial bleeding, leptomeningeal disease, mass lesion; lumbar puncture may detect malignant cells
FDG-PET/CT scan
Test
Should be considered in patients with suspected extramedullary disease.[45]
Result
may show extramedullary lesions
chest x-ray
Test
May be performed to identify pneumonia, mediastinal masses, pulmonary infiltrates, or cardiomegaly.
Result
may show evidence of pneumonia, mediastinal masses, pulmonary infiltrates, cardiomegaly
echocardiogram
multi-gated acquisition scan
human leukocyte antigen (HLA) typing
Test
Ordered to assess and match for suitable donor for allogeneic stem cell transplant.[45]
Result
variable
Use of this content is subject to our disclaimer