Details
Infant care during the first year of life is of particular importance. During this period many neurodevelopmental, metabolic, and physical disorders become apparent. Abnormalities recognized and promptly addressed may improve or at least preserve overall function and quality of life. Anticipatory guidance, addressing parental concerns, and maintaining appropriate levels of immunizations are the priority in well infant exam care. Early childhood home-visiting services can improve child health and family well-being: the American Academy of Pediatrics (AAP) recognizes home visits as an integral part of a comprehensive early childhood system.[1]
Parental expectations regarding care of a well infant range from a short conversation answering specific questions or concerns to a complete examination, screening tests, and age-specific immunizations.[2] Although there is no direct evidence of benefit from repeated physical exams, they are routine in most US pediatric practices.[3][4][5]
The age-specific recommendations and priorities included are based on current "Bright Futures" recommendations from the AAP, which encourage the most comprehensive level of screening and management.[4] Additional recommendations from the Cochrane databases and other organizations such as the American Academy of Family Physicians (AAFP), the Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO), and the US Preventive Services Task Force (USPSTF) are also included:
CDC: infants and toddlers (approximate ages 0-3) Opens in new window
USPSTF: pediatric recommendations Opens in new window
WHO: improving early childhood development Opens in new window
Newborn physical assessment includes:[4][6][7]
[Figure caption and citation for the preceding image starts]: Newborn physical assessmentCreated by the BMJ Knowledge Centre [Citation ends].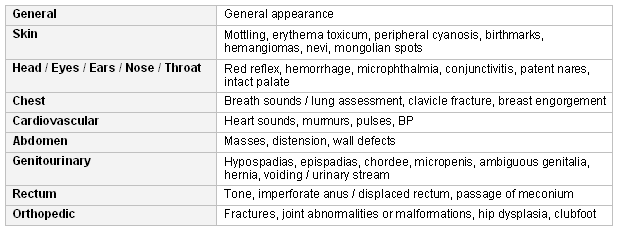 The goals of well-child examinations are to maximize development, detect treatable diseases early, and provide information. Most of the assessment can be done while taking a history from the parent and by observing the child. Improvements in surveillance tools and processes could help to identify concerns, support developmental screening decisions, and allow timelier referral to early intervention.[8]
The goals of well-child examinations are to maximize development, detect treatable diseases early, and provide information. Most of the assessment can be done while taking a history from the parent and by observing the child. Improvements in surveillance tools and processes could help to identify concerns, support developmental screening decisions, and allow timelier referral to early intervention.[8]
[Figure caption and citation for the preceding image starts]: Recommendations for screening examinations at various ages in infancyCreated by the BMJ Knowledge Centre [Citation ends].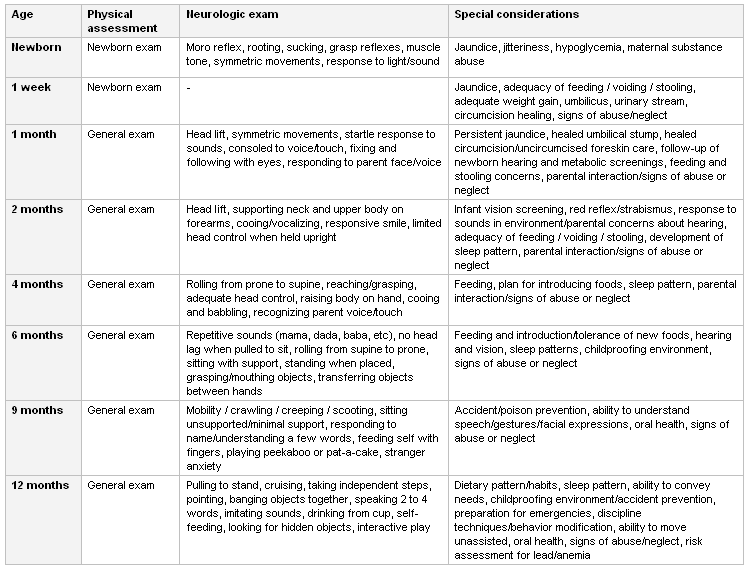
Routine blood sample-based newborn screening is performed on a near universal basis in developed countries and has significantly decreased potential morbidity and mortality of a wide range of inborn metabolic and some genetic disorders among infants.
Newborn screening is mandatory in the US and is state-based.[9] All 50 states screen neonates for congenital hypothyroidism, galactosemia, and phenylketonuria (PKU). Routine screening typically includes at least some of the following, with variation between states:[9] HRSA: recommended uniform screening panel Opens in new window
Amino acid metabolism disorders
Biotinidase deficiency
Congenital adrenal hyperplasia
Congenital hypothyroidism
Cystic fibrosis
Fatty acid metabolism disorders
Galactosemia
Glucose-6-phosphate dehydrogenase deficiency (G6PD)
Human immunodeficiency disease (HIV)
Organic acid metabolism disorders
Sickle cell disease and other hemoglobinopathy disorders and traits
Toxoplasmosis.
As detection thresholds are intentionally at the lower range of a potentially abnormal result, the potential for false positive result may be elevated in some screening assays. Abnormal results should be promptly addressed with repeat testing of the abnormal result and appropriate specialty evaluation expeditiously arranged. In view of variation in the newborn screening panels between states, it is important for the individual practitioner to remain aware of which conditions are included on a particular state neonatal assay.
Although specific recommendations for screening are addressed at various ages, it remains the decision of the individual practitioner to weigh the risks/benefits associated with a particular screening intervention, such as a false positive result or any morbidity from follow-up, with a late detection/diagnosis.[10][11][12][13][14]
In the US, 1 in 6 children ages 13 to 17 years are diagnosed with a developmental disability.[15] Because early intervention services can have a significant impact on a child’s long-term outcomes, the AAP recommends that developmental surveillance (longitudinal monitoring) aimed to identify children at risk for developmental delay occurs during every health supervision visit.[16] This surveillance, coupled with formalized screening using validated tools, is the best available strategy for timely identification of delays and referral for early intervention services.[16] The CDC have partnered with the AAP to convene an expert working group to revise and update its developmental surveillance checklists for pediatric providers.[8][17] The CDC “Learn the Signs. Act Early” program facilitates parental engagement in milestone tracking.[17] Their publicly-available toolkit includes developmental milestone checklists, a Milestone Trackers App, and videos to engage families and encourage ongoing conversations with their child’s pediatric healthcare providers.
The WHO provides a number of recommendations in improving early childhood development in all infants. WHO: improving early childhood development Opens in new window
In the US, hearing loss is the most commonly diagnosed condition by newborn screen, with about 5000 infants born every year with permanent moderate to profound bilateral hearing loss.[9] The estimated incidence of permanent congenital hearing loss is 1/3000 live births.[18][19] Risk factors associated with a higher incidence of permanent hearing loss include neonatal intensive unit admission >5 days, family history of permanent childhood hearing loss, craniofacial anomalies, in-utero infections (e.g., cytomegalovirus), physical findings of a syndrome or diagnosis of a syndrome known to include hearing loss, neurodegenerative disorders, postpartum infections (e.g., meningitis), and head trauma.[4] However, often diagnosis and treatment are delayed until ages 1 to 2 years, particularly in those who are deemed to be at low risk.[20][21] Hearing loss is attributed as a cause for many developmental morbidities, particularly communication, cognition, reading, and social-emotional development.[22][23]
To maximize the developmental potential for infants who are deaf or hearing impaired, all infants should undergo hearing screening prior to discharge from the birth hospital and no later than 1 month of age, using physiologic measures with objective determination of outcome, and this is now mandated in most states.[4][18][24][25] The WHO recommends screening all newborns using otoacoustic emissions (OAE) or automated auditory brainstem response (AABR) to promptly detect permanent bilateral hearing loss.[26] Newborns found to have a hearing deficit at screening should receive a comprehensive audiologic evaluation before 3 months of age, and appropriate intervention before 6 months of age if the hearing deficit persists.[27][28] Regardless of the outcome from previous screenings, all infants, with or without risk factors, should be monitored for communication development during well-child visits beginning at 2 months of age.
Data from poor-quality studies suggest that universal infant hearing screening is highly effective in detecting hearing impairment, with sensitivities and specificities ranging between 50% and 100%.[29][30][31] Because the middle ear indices change with maturation from fetal stage to young adolescence, infant hearing screening methods with a higher degree of sensitivity need to be identified.[32][33]
Although difficult to accomplish, measurement of infant visual acuity is possible. Serious vision impairment and blindness in infants must be detected as early as possible.[34]
Visual impairment is common in young children, estimated to affect 5% to 10% of all preschoolers. Because an early "sensitive period" can help early intervention, prompt and early detection, referral, and treatment at the primary care level is mandatory.[4]
Causes of serious visual impairment and blindness may originate in the prenatal, perinatal, and postpartum periods and must be promptly recognized. Impairment at birth is typically a result of congenital anomalies such as microphthalmos, anophthalmos, coloboma, congenital cataracts, infantile glaucoma, and neuro-ophthalmic lesions. Perinatally acquired causes include retinopathy of prematurity (ROP), ophthalmia neonatorum, and cortical visual impairment.
ROP, a complex condition of the developing retinal blood vessels, is one of the leading preventable causes of childhood blindness. Multiple risk factors have been studied, including general immaturity and prolonged oxygen therapy. Progression is multifactorial and possibly associated with other risk factors. The WINROP clinical algorithm utilizes weight and insulin-like growth factor (IGF)-1 to detect preterm infants at risk of severe ROP. Compared with results of actual (direct) ROP screening, the WINROP algorithm provides a noninvasive method for identifying infants at high risk of severe ROP as well as those who are not at risk.[35] A study examining the relationship between the cause/severity of hypotension in infants at <28 weeks' gestation and development of severe ROP found low cortisol levels in the presence of dopamine-resistant hypotension had a high magnitude of association with severe ROP and likely accounted for the relationship of severe ROP with "idiopathic" hypotension.[36] One study demonstrated significantly reduced odds of ROP in low birth weight survivors with trisomy 21, which may unmask a potentially identifiable genetic ROP risk component, and may eventually contribute to a laboratory-based screening tool for ROP.[37]
The AAP published guidance in 2018 that recommended a program to detect and treat ROP; it suggested that substitution of algorithms (such as WINROP) for screening measures was not justified by the existing evidence base.[38] The AAP screening program recommends that all infants with a gestational age of ≤30 weeks, or a birth weight of ≤1500 g should be screened. Other infants at risk for ROP should be also be screened, including those with a birth weight between 1500 to 2000 g or a gestational age of >30 weeks, and who have had hypotension requiring inotropic support, oxygen supplementation for more than a few days, or oxygen without saturation monitoring. Detailed criteria for initial exam, follow-up, and treatment are provided. [39]
Leukocoria (white pupillary reflex) may indicate congenital cataract, persistent hyperplastic primary vitreous, or retinoblastoma. Although few surgical or medical options beyond adaptive care such as low vision aids and rehabilitation are available for congenital anomalies or neuro-ophthalmic disorders, surgery for congenital cataracts should occur within the first 4 months of life to achieve greatest potential benefit.[34]
The USPSTF reports that screening tests have reasonable accuracy in identifying strabismus, amblyopia, and refractive error in children ages 3 to 5 years with these conditions, with no evidence of harm. The balance of benefits and harms are uncertain in children younger than 3 years. They conclude that early detection and treatment of these conditions can improve visual acuity and reduce long-term amblyopia. As such, the USPSTF recommends screening to detect amblyopia or its risk factors (strabismus, refractive errors, and media opacity) in children ages 3 to 5 years.[40] The AAP, the American Academy of Ophthalmology, and the American Association For Pediatric Ophthalmology and Strabismus strongly support vision assessment from birth onward. AAO: vision screening for infants and children - 2022 Opens in new window Recommendations include a detailed ocular history, vision assessment (ability to fix and follow), external inspection of eyes and eyelids, ocular motility assessment, pupil exam, and red reflex exam.[41] AAO: vision screening for infants and children - 2022 Opens in new window The cover test and Hirschberg light reflex are typically used to screen for strabismus. Newer automated photoscreening methods can detect amblyogenic risk factors such as strabismus, media opacities, and significant refractive errors, but they cannot specifically detect amblyopia.[34][40]
All children with ocular abnormalities or failed vision screening, and any high-risk child, should be referred to an a clinician experienced in treating children for a specialized eye exam.[41] AAO: vision screening for infants and children - 2022 Opens in new window Children deemed high risk include extremely premature newborns and those with metabolic or genetic disorders, significant developmental delay or neurologic disorder, and systemic disease associated with eye abnormalities, children with any opacity of the ocular media, children with nystagmus, or children a positive family history of congenital cataract, retinoblastoma, glaucoma in childhood or retinal dystrophy/degeneration.[34][41] AAO: vision screening for infants and children - 2022 Opens in new window
The Advisory Committee on Immunization Practices (ACIP) of the CDC recommend that health providers should support and implement standards for infant immunization practices. These practices represent expert opinion on scientific evidence of the balance of benefits and harms from immunizations. The Recommended Childhood Immunization Schedule is revised annually. CDC: child and adolescent immunization schedule by age Opens in new window
Immunization for vaccine-preventable diseases that should be initiated during the first 15 months of a child’s life include respiratory syncytial virus, hepatitis B, rotavirus, diphtheria, tetanus, pertussis, polio, pneumococcal disease, Haemophilus influenzae type B (Hib), influenza, and COVID-19. At 12 months, measles/mumps/rubella (MMR), hepatitis A, and varicella vaccines are recommended. CDC: child and adolescent immunization schedule by age Opens in new window
The ACIP recommends simultaneously giving all vaccines for which a child is eligible.[42] Immunization should not be delayed due to mild upper respiratory tract infections, as it can lead to missed opportunities. National standards for pediatric immunization practices have been established and include genuine contraindications (e.g., severe allergy to previous dose of vaccine) and precautions (e.g., moderate to severe acute disease with or without fever) to immunization.[43]
Premature infants have increased susceptibility to infectious diseases, of which some are vaccine-preventable. Maturation of immune response for all infants begins with exposure to antigens in the environment and occurs at a similar rate in term and premature infants; thus, premature infants should begin immunizations at 2 months of (chronologic) age regardless of actual gestational age. For infants with low birth weight, vaccine response can be lower; therefore, if the infant is <2 kg birth weight, their subsequent hepatitis B vaccination schedule may include an extra dose.[44]
Studies have found an increased risk of apnea and/or bradycardia within 48 to 72 hours following immunization of infants with gestational age <32 weeks. The risk appears highest with use of DTP-IPV-HIB vaccine, although DTaP-IPV-HIB vaccine seems to have approximately the same degree of incidence. Lower weight at time of immunization was also identified as a risk factor, as was extreme low birth weight and infants previously experiencing an episode of sepsis. Considering the increased level of risk and potential for acute life-threatening events in this patient population, it would be appropriate to monitor cardiorespiratory status during the 72 hours post-immunization.[45][46][47]
Vitamin K supplementation in the neonatal period
Although vitamin K deficiency, a disorder of early infancy resulting from low concentrations at birth, is rare in developed countries, the potentially life-threatening consequences provide the impetus for routinely giving prophylactic vitamin K at birth. The classic vitamin K deficiency bleeding (VKDB) occurs within the first week of life. It is idiopathic, although often thought to result from delayed or inadequate dietary intake; breastfed infants are at greater risk due to low breast milk vitamin K concentrations. It is readily preventable by small doses of vitamin K at birth. Late vitamin K deficiency is less easily recognized or prevented, and frequently presents as intracranial hemorrhage, with a peak incidence at 3 to 8 weeks of age. It typically results from vitamin K malabsorption.[48][49][50] Phytonadione (1 mg intramuscularly) provides almost complete protection against bleeding due to vitamin K deficiency, both classic and late forms, in exclusively breastfed infants.[48][49][50][51] Previously recommended intramuscular doses ranging between 0.5 and 1.0 mg or single oral doses of 1 to 2 mg are not effective. Omission of prophylaxis, its use in selected high-risk infants, or the use of oral prophylactic therapy, after concerns that intramuscular vitamin K prophylaxis may be unnecessary or potentially toxic, have resulted in disastrous outcomes.[48] If the oral route is preferred for newborn prophylaxis, a dosing regimen of 1 mg at weekly intervals for 12 weeks or 2 mg at weeks 1 and 4 should be considered. Protection provided at a dose of 25 micrograms daily is insufficient.[48][50] Infants of mothers receiving medications that inhibit vitamin K are at increased risk of early VKDB and should receive phytonadione 1 mg intramuscularly as soon as possible after birth.[48][50]
Vitamin A and vitamin D supplementation in infancy
Micronutrient deficiencies are common, even in higher-income countries. Although clear proof of a benefit of individual micronutrient supplementation to child growth is lacking, multivitamin supplementation during the ages of 3 to 24 months has a beneficial effect on growth in children highly compliant with treatment.[52][53] The AAP recommends vitamin D supplementation of 400 IU/day soon after birth in healthy term infants that are exclusively breastfed, or who receive both breast milk and formula.[54][55] As infant formula is fortified with vitamin D, additional supplementation is not necessary for infants exclusively fed on formula.[54][55] Similarly, the Canadian Paediatric Society recommends all exclusively breastfed healthy term infants receive 400 IU/day vitamin D supplement until the diet includes at least that amount from other sources, with an increase in intake level to 800 IU/day in northern native (Inuit) communities during winter months.[56][57] The rationale for this is drawn from studies of vitamin D deficiency in adults, defined as a 25-hydroxy vitamin D (25-OH-D) concentration of <50 nanomols/L.[58][59][60] Whether these levels are insufficient for children is unclear. However, studies show that supplementation with 200 IU/day does not maintain 25-OH-D levels >50 nanomols/L, but supplementation at 400 IU/day does.[54] Daily supplementation of 800 IU vitamin D in preterm infants 28 to 34 weeks' gestation reduces the incidence of vitamin D deficiency at 40 weeks' postmenstrual age and 3 months' chronologic age. However, there is the possibility of vitamin D excesses.[61] Despite a large number of systematic reviews and meta-analyses, highly convincing evidence for any outcome does not demonstrate a clear role for vitamin D although there are probable associations.[62][63] Brief daily ultraviolet (UV) exposure causes negligible skin damage while stimulating vitamin D production; however, attempting a further increase by extended solar UV exposure or solarium (UVA) irradiation is inadvisable.[64] The role of routine vitamin A supplementation is less clear. Vitamin A supplementation has not reduced overall morbidity or mortality in developing countries in infants younger than 6 months, although no adverse effects of supplementation were noted.[65][66][67][68] [
 ]
[
]
[  ]
]
There is evidence to suggest a decrease in morbidity/mortality and a reduced incidence of vision disorders in children ages 6 months to 5 years in developing countries.[69][70][71] However, in the US, early vitamin supplementation, specifically vitamins A and D, may be associated with asthma in black children and food allergies in exclusively formula-fed infants.[72] The AAP suggests that preterm infants should receive both a multivitamin preparation and an oral iron supplement until they are ingesting a completely mixed diet and their growth and hematologic status are normalized.[73] The WHO does not recommend routine vitamin A supplementation to reduce neonatal and infant mortality.[26]
Although not a direct neonatal therapy, a study of vitamin C supplementation of 500 mg/day in smoking pregnant women found an improved newborn pulmonary function test result and decreased wheezing through the first year of life compared with infants of untreated smoking mothers and a cohort of infants born to nonsmoking mothers. However, this effect was not found to be sustained at a level of significance beyond the first year of life.[74]
Iron supplementation
The neonate initially depends on a maternal source for iron stores.[75][76] There is evidence that delayed cord clamping increases early hemoglobin concentrations and iron stores in infants.[77] [
 ]
Iron deficiency before 6 months of life, although uncommon (3% incidence), can occur. Iron supplementation to exclusively breastfed infants from 1 month of age (7 mg/day) increases serum ferritin levels but not hemoglobin.[78] All commercially available US infant formulas are iron-fortified, although actual levels vary between products. Routine iron supplementation during the first year of life should be based on an infant-specific judgment of need.[79] Iron supplementation in preterm and low birth weight infants has been shown to improve hematologic indicators of iron status and decrease incidence of iron-deficiency anemia; however, there is insufficient evidence regarding the long-term impact on growth and neurodevelopment, or on the potential to cause adverse effects in preterm and low birth weight infants.[80]
[
]
Iron deficiency before 6 months of life, although uncommon (3% incidence), can occur. Iron supplementation to exclusively breastfed infants from 1 month of age (7 mg/day) increases serum ferritin levels but not hemoglobin.[78] All commercially available US infant formulas are iron-fortified, although actual levels vary between products. Routine iron supplementation during the first year of life should be based on an infant-specific judgment of need.[79] Iron supplementation in preterm and low birth weight infants has been shown to improve hematologic indicators of iron status and decrease incidence of iron-deficiency anemia; however, there is insufficient evidence regarding the long-term impact on growth and neurodevelopment, or on the potential to cause adverse effects in preterm and low birth weight infants.[80]
[  ]
One study found significant improvement in serum ferritin and hemoglobin levels at 12 weeks' postpartum age in preterm, very low birth weight infants commencing early iron supplementation.[81]
]
One study found significant improvement in serum ferritin and hemoglobin levels at 12 weeks' postpartum age in preterm, very low birth weight infants commencing early iron supplementation.[81]There is some evidence of a mild positive effect of iron supplementation on cognitive and psychomotor outcomes following a minimum of 2 months' use in anemic infants and children.[82] Mild iron deficiency in infancy is thought to be a factor in subsequent development of behavioral problems in low birth weight infants. Some data also suggest iron supplementation during the first 6 months of life in low birth weight infants reduces the risk of behavioral problems at age 3.5 years.[83][84] The AAP recommends that all infants should receive iron-fortified formulas or iron supplementation and that screening for anemia should occur at 12 months of age by determination of hemoglobin concentration.[4] The use of high-dose recombinant erythropoietin for neuroprotection for anemia in very premature infants is safe and demonstrates no excess in mortality or major long-term adverse effects at follow-up visits.[85]
Zinc supplementation
Zinc deficiency is thought to be present in 30% of the world's population, with the highest prevalence in children younger than 5 years in developing countries. Associated with immune system dysfunction leading to infection-related morbidity, growth retardation, hypogonadism, and cognitive dysfunction, zinc deficiency is primarily related to the consumed diet, with zinc being most abundant and readily absorbed from animal proteins; consumption of vegetable proteins and cereals, with binding of zinc to phytates, results in decreased absorption. Strong evidence has developed over the past two decades showing a positive effect of zinc in reducing morbidity and mortality in children due to gastrointestinal and respiratory infections. Most studies have found benefit from supplementation in the daily range of 10 to 20 mg and duration >3 months, although there is some disagreement among studies.[86][87][88][89] One review suggests the benefits of preventive zinc supplementation may outweigh the harms in regions with high levels of zinc deficiency.[90] Zinc supplementation has shown positive effect on linear growth in children younger than 5 years.[86][87]
Micronutrient fortification of milk and cereal food
Data also support multimicronutrient fortification of milk and cereal products providing a synergistic effect on hematologic outcomes over singular iron fortification in children up to 3 years of age in developing countries. Similarly, fortified foods appear to show increased serum vitamin A levels, but not zinc, over nonfortified foods; however, data remain inconclusive regarding evidence of functional health outcomes.[91]
Fluoride supplementation
The American Academy of Pediatric Dentistry recommends optimum levels of fluoride in community water supplies. Fluoride supplementation reduces dental caries and can reverse enamel demineralization. US and Canadian ready-to-feed formulas are only modest sources of supplemental fluoride, in the range of 0.1 to 0.3 mg/L, while non milk-based formulas contain a higher level due to the fluoride content of the calcium added to achieve levels equivalent to milk-based products. A fluoride supplement for infants over 6 months of age is recommended only after considering the fluoride levels ingested from dietary sources.[92][93]This is because fluorosis, or excessive fluoride ingestion, may follow the use of community or bottled water to reconstitute powdered formulas, and is a greater concern.
Supplements in children with HIV
Vitamin A supplementation is beneficial and safe in children with HIV infection. Zinc is safe and appears to have similar benefits on diarrheal morbidity in children with HIV as in children without HIV infection. Multiple micronutrient supplements have some clinical benefit in poorly nourished children with HIV infection. Further trials of single supplements (vitamin D, zinc, and selenium) are required. The long-term effects and optimal composition and dosing of multiple micronutrient supplements requires further investigation.[94]
[  ]
[
]
[  ]
[
]
[  ]
[
]
[  ]
]
Docosahexaenoic acid (DHA) supplementation
There has been much ongoing debate regarding the efficacy of DHA supplementation on infant development. A randomized controlled study compared a 6-month course of microencapsulated DHA with arachidonic acid to placebo (microencapsulated corn oil) in infants born at <35 weeks' gestational age. The study evaluated infants who were 10 to 16 months’ corrected gestational age and demonstrated no significant differences between groups utilizing the Bayley Scales of Infant and Toddler Development, third edition (Bayley III). Neither the primary outcome of cognitive composite score at 16 to 22 months’ corrected age nor the secondary outcomes of language and motor composite scores demonstrated any benefit. Of particular concern was the finding of a small to medium negative effect on Bayley III language scores among children with low birth weight.[95]
Although the USPSTF has found there to be insufficient evidence to recommend for or against routine blood pressure screening in children, the AAP recommends selective screening of infants with specific risk factors such as:[4]
History of prematurity, low birth weight, or other neonatal condition requiring intensive care
Congenital heart disease (repaired or nonrepaired)
Recurrent urinary tract infections, hematuria, or proteinuria
Known kidney disease/urologic malformation
Family history of congenital kidney disease
Solid organ transplants
Malignancy
Treatment with medications known to increase blood pressure
Systemic illness associated with hypertension (i.e., neurofibromatosis, tuberous sclerosis)
Evidence of elevated intracranial pressure.
Transient tachypnea of newborn
Transient tachypnea of the newborn (TTN), resulting from delayed clearance of lung fluid, is particularly common after elective cesarean section delivery. Conventional treatment involves appropriate oxygen administration and, on occasion, continuous positive airway pressure with most infants receiving empiric antibiotic therapy. Furosemide is not effective in promoting resorption of lung fluid, or factors other than delayed fluid resorption contribute to the pathogenesis of TTN.[96] [
 ]
]
Hypothermia
Environmental hypothermia: the incidence of hypothermia (axillary temperature <96.8ºF [<36ºC]) is 44% at 5 minutes after birth and 51% on admission to the neonatal intensive unit (NICU), and is associated with 6% of early neonatal deaths.[97] The use of simple interventions, such as delivery room ambient temperature >77ºF (>25ºC), use of plastic bags/wraps and caps to minimize room air exposure of newly born infants, and warming of resuscitation gases will potentially decrease hypothermia incidence and improve early neonatal survival.[98] In one study, use of the exothermic mattress in conjunction with the polyethylene bag resulted in fewer infants having temperatures within the desired target range and an increased incidence of hyperthermia on NICU admission.[99] In a multicenter, open-label, randomized control trial comparing conductive thermal mattress (CTM) with the standard intervention of radiant warmers and blankets in a resource-limited setting, CTM was found noninferior.[100]
Therapeutic hypothermia: therapeutic hypothermia has now become the standard of care in the management of hypoxic-ischemic encephalopathy in resuscitated infants and has been found to improve survival and neurodevelopmental outcomes when started within 4 hours of delivery. There is no current consensus on how best to manage infants requiring therapeutic hypothermia during transport. One study comparing passive cooling (while awaiting arrival of transport team) with active cooling with a servo-controlled mattress found the latter group of infants to have a better target temperature at arrival and reduced transfer time.[101]
Pain management
With the growing recognition and emphasis on procedural pain in neonates, various pain management interventions (sucrose, analgesics, rocking, and skin-to-skin contact) have been studied.[102][103][104][105] [
 ]
Skin-to-skin contact was shown to be more effective than oral glucose or dextrose, but various combinations were more effective than single interventions, and skin-to-skin contact was shown to be as effective as breast-feeding.[102] One study in preterm infants <30 weeks' gestational age found that therapeutic touch, given before and after heel lance, had no comforting effect in this infant population and other alternative strategies utilizing actual touch should be considered.[106] One study found the use of combined radiant heat with oral sucrose for control of pain during infant immunization resulted in decreased physiologic markers of pain and distress with approximately 50% decrease in duration of crying/grimacing.[107]
]
Skin-to-skin contact was shown to be more effective than oral glucose or dextrose, but various combinations were more effective than single interventions, and skin-to-skin contact was shown to be as effective as breast-feeding.[102] One study in preterm infants <30 weeks' gestational age found that therapeutic touch, given before and after heel lance, had no comforting effect in this infant population and other alternative strategies utilizing actual touch should be considered.[106] One study found the use of combined radiant heat with oral sucrose for control of pain during infant immunization resulted in decreased physiologic markers of pain and distress with approximately 50% decrease in duration of crying/grimacing.[107]One Cochrane review of trials using acetaminophen for control of neonatal pain found no adverse effects for use in neonates; however, it was not found to significantly reduce pain associated with eye exams or heel lance. In addition, use of acetaminophen for pain control following assisted vaginal birth may increase the response to later painful exposures. Acetaminophen, by itself, does not provide adequate pain control for significant painful procedures and should not be used for that purpose, although it may reduce the total morphine needed following major surgery.[108]
Newborn skin care
Development of the skin barrier in infants continues throughout the first 12 months of life.[109] Specific considerations during this initial period include the following:
Loose adherence of the epidermis to dermis, resulting in easy blister formation during inflammatory or irritant processes:
More likely to react to irritants
Prone to maceration as a result of moisture retention
Increased transdermal water loss
Higher percutaneous absorption of applied substances.
Epidermal barrier incompletely developed, resulting in increased susceptibility to microbial attack
Lower epidermal melanin content, allowing increased susceptibility to ultraviolet (UV) light-induced damage
Increased tendency to dyshidrosis and damage due to disturbance of the epidermal barrier and protective acid mantle by soaps and cleansers.[110]
For these reasons, certain principles of infant skin care take high priority, such as: gentle cleansing, adequate hydration or moisturizing of the skin, preventing friction and/or maceration in skin fold areas, and protection from irritants and excessive sunlight or other UV light sources.[109][110][111]
The skin's barrier function resides primarily within the epidermal stratum corneum layers and consists of keratinocytes. Another class of lipids also secreted at the epidermal surface interacts with water to form a hydrophilic film, which plays a major role in maintaining skin moisture content.[110]
The "acid mantle" is the functional capacity of the skin to form a surface pH <5, which is important to maintain as there is a close association between skin pH and microbial flora, with an increase of pH from acid to neutral causing a transient increase in total number present and shift in species of skin bacteria present.[110] Regeneration of skin pH after washing with alkaline soaps requires at least 1 hour, and thus alkaline soaps should not be used in the first months of life.[64]
Bathing of the newborn is the ideal means of cleansing to remove blood and vernix as well as decreasing exposure to maternal blood and the inherent risk of hepatitis and HIV viruses. For the first few weeks of life, bathing in lukewarm water (<99°F [<37°C]) is usually adequate and soaps or cleansers are best avoided.[110] The bulk of most cleansers consists of a surfactant agent, which works by decreasing surface tension between water and air, creating a foaming action that allows for removal of fat-soluble impurities from the skin. Agents with higher foaming power increase the potential for excessive removal of stratum corneum lipids, resulting in skin damage.[110]
Emollient agents, frequently referred to as moisturizers or lubricants, serve to soften and smooth the skin and are composed of lipids, which may be of organic (animal or plant), mineral oil, or synthetic (hydrocarbon) origin. There are currently no data that support efficacy of emollient agents for the prevention of invasive infections in preterm infants regardless of socioeconomic setting. By inference, the study would suggest a similar lack of such beneficial effect in term infants as well. There is low-to-moderate certainty evidence that emollients in healthy infants are not effective in preventing eczema, may increase the risk of food allergy, and may increase risk of skin infection.[112][113] There is limited evidence of positive effect for some vegetable oils on neonatal growth; however, the results were obtained via a small unblended study with potential for caregiver and assessor bias. The findings should therefore be viewed with caution.[114][115] [
 ]
The WHO does not recommend routine application of topical emollients in term, healthy newborns for the prevention of skin conditions.[26]
]
The WHO does not recommend routine application of topical emollients in term, healthy newborns for the prevention of skin conditions.[26]If cleansing agents are truly required, healthy newborns and infants should be washed with cleansers of neutral to slightly acidic pH that utilize a gentle surfactant and should contain an emollient.[110]
Umbilical cord care
Increasingly strong data supporting delayed cord clamping in newborns (ideally after cessation of cord pulsation, but at least 60 seconds post birth) is becoming available.[116] Evidence demonstrates an increase in infant hemoglobin with lower incidence of iron-deficiency anemia at 4 to 6 months of age, and does not significantly increase the incidence of neonatal jaundice.[77][117][118][119][120] [
 ]
The addition of umbilical cord milking/stripping (UCM) in a preterm infant prior to clamping does not demonstrate any significant advantage over delayed cord clamping 60 to 90 seconds post delivery, and the Canadian Pediatric Society does not recommend UCM in very preterm infants <32 weeks due to increased risk for severe intraventricular hemorrhage.[116][121][122] However, there are some data suggesting this may create a higher systemic blood flow with resulting decreased need for transfusion and incidence of intraventricular hemorrhage (IVH).[123][124] Similarly, there is evidence that establishment of ventilation (spontaneous or assisted) prior to cord clamping is also likely to decrease the incidence of IVH and improve physiologic stability in the newborn.[125] Delayed cord clamping should be performed instead of UCM in preterm and term infants.[116] Delayed cord clamping in infants with critical congenital heart disease, in limited studies to date, also appears safe and feasible.[126][127][128] Limited data in a low-risk population born in a high-income country demonstrated improved scores in the fine motor and social domains, particularly in males, at 4 years of age in infants who had a delay in umbilical cord clamping and warrants further study in a more diverse population.[129]
]
The addition of umbilical cord milking/stripping (UCM) in a preterm infant prior to clamping does not demonstrate any significant advantage over delayed cord clamping 60 to 90 seconds post delivery, and the Canadian Pediatric Society does not recommend UCM in very preterm infants <32 weeks due to increased risk for severe intraventricular hemorrhage.[116][121][122] However, there are some data suggesting this may create a higher systemic blood flow with resulting decreased need for transfusion and incidence of intraventricular hemorrhage (IVH).[123][124] Similarly, there is evidence that establishment of ventilation (spontaneous or assisted) prior to cord clamping is also likely to decrease the incidence of IVH and improve physiologic stability in the newborn.[125] Delayed cord clamping should be performed instead of UCM in preterm and term infants.[116] Delayed cord clamping in infants with critical congenital heart disease, in limited studies to date, also appears safe and feasible.[126][127][128] Limited data in a low-risk population born in a high-income country demonstrated improved scores in the fine motor and social domains, particularly in males, at 4 years of age in infants who had a delay in umbilical cord clamping and warrants further study in a more diverse population.[129]The risk of omphalitis is more common in developing countries than in the US.[130][131] Several studies have found no superior agent in umbilical cord care, making comprehensive recommendations difficult.[132][133] Among potential cord care antiseptics, chlorhexidine is superior to ethanol, povidone-iodine, and hexachlorophene in reducing cord group A Streptococcus and Staphylococcus aureus, but not coagulase-negative Staphylococcus or enteric gram-negative bacilli. Triple dye is superior to isopropanol, hexachlorophene, and bacitracin in controlling bacterial cord colonization.[134][135][136] A single application of triple dye reduced cord staphylococcal colonization but promoted growth of group B beta-hemolytic Streptococcus and gram-negative organisms, a problem that resolved with daily applications.[132]
Cord care regimens may prolong time to cord separation, possibly by decreasing bacterial colonization and limiting leukocyte influx to the cord stump, although prolonged cord separation seems to reduce the risk of cord infection.[130][133][136] Risk of toxicity is highest with hexachlorophene and povidone-iodine and should be avoided in preterm infants. Toxicity from triple dye is rare.[134]
Until adequate controlled prospective studies identify the most efficacious agent to reduce neonatal sepsis or omphalitis risk, chlorhexidine provides the most effective antisepsis with the fewest potential adverse effects.[134][136][137][138]
Trials of “dry” cord care, which varies in actual process, suggest this may be safe in infants born in developed countries, and is recommended by WHO.[26] However, all studies highlight the lack of a consensus on management approach as well as specific, broadly accepted guidelines for neonatal umbilical cord care.[139][140][141]
Teething is a frequent source of discomfort in infancy, and may be a significant source of parental concern. Many remedies have varied levels of success; however, cold (but not frozen) hard rubber teething rings or rubbing the gums with a clean finger are considered the safest options. AAP: how to help teething symptoms without medications Opens in new window Older children may also experience discomfort as permanent dentition develops. A warning from the Food and Drug Administration (FDA) draws attention to the increasing popularity of teething necklaces, bracelets, and anklets made from materials such as marble, silicon, and amber being used to relieve pain and offer sensory stimulation to children with autism or other developmental delays. Some manufacturers also suggest therapeutic benefit from the purported anti-inflammatory effect of succinic acid released from amber beads.[142] Although succinic acid is an intermediate in several metabolic pathways and has demonstrated some bacteriostatic properties against Escherichia coli and other organisms, it is known to irritate skin, eyes, and mucous membranes, particularly oral and esophageal tissues. As the amount of succinic acid released is unpredictable and reliable assays to determine levels do not exist, the risk of toxicity from teething jewellery may be significant. Additionally, aspiration of saliva with soluble succinic acid may have severe pulmonary consequences. Teething jewelry also presents a significant choking and strangulation risk, while extremity entrapment with associated degloving injury and tourniquet effect has a high level of life-altering sequelae.[143] The FDA advises against teething creams, benzocaine gels, sprays, ointments, solutions, and lozenges, which can result in potentially life-threatening methemoglobinemia. Topical application of alcohols such as bourbon, rum, vodka, and other liquors/liqueurs is also contraindicated due to potential toxicity from systemic ethanol absorption. Commercially available teething tablets should be evaluated with caution as some homeopathic products contain compounds such as belladonna, lead, or arsenic. US FDA: laboratory analysis of homeopathic teething tablets Opens in new window Unregulated agents may not contain consistent quantities of the various ingredients.
There are no high-quality studies evaluating accurate identification of children with one or more risk factors for oral disease. Routine guidance against high-sugar-content foods and juices, as well as prevention of nursing-bottle caries, should be addressed during routine health maintenance visits. The AAP and the USPSTF recommend referral based on risk assessment by the primary healthcare provider to an oral healthcare provider as early as 6 months, within 6 months of eruption of the first tooth, or no later than 12 months of age.[4][144][145] There is a recommendation for application of fluoride to children’s teeth from 1 year of age; this has not been evaluated in infants ages <1 year. In the case of infants ages 6 months or older, whose water supply is deficient in fluoride, it is recommended that oral fluoride supplementation be prescribed by the primary care clinician.[4] Caution should be exercised to avoid excessive fluoride intake and avoid complications such as oral fluorosis and osseous changes.[146]
Sleep patterns rapidly evolve in infancy. A newborn averages 16 hours of sleep divided over multiple, relatively short episodes during a 24-hour period. With the development of a circadian pattern over the first 6 months, nocturnal sleep duration gradually extends and consolidates, while daytime sleep diminishes. This subsequently evolves, over the first year of life, into a more consolidated, predominantly nocturnal sleep pattern. However, many infants continue to awaken two to three times during the night.
An infant's inability to resume sleep without parental involvement and/or awareness is one of the most common complaints made by parents.[147] Other highly prevalent sleep problems in infancy are night awakenings and sleep-disordered breathing. Most problems of infant night awakening seem to have a behavioral or developmental etiology and typically resolve with simple interventions such as maintaining a consistent, stable bedtime ritual and limiting active parental involvement and/or physical contact in the process of sleep onset. Sleep-disordered breathing is more often associated with medical and developmental disorders.[147]
Disruption of the normal progression of nonrapid eye movement sleep may also result from iron-deficiency anemia.[148] This seems to correlate with the high frequency of poor sleep patterns among poor, immigrant, and minority children and should be considered in the evaluation and management of infant sleep problems.
In a study measuring sleep parameters by actigraphy and polysomnography, patients with atopic dermatitis were found to exhibit significantly reduced sleep efficiency, longer sleep-onset latency, more sleep fragmentation, and less nonrapid eye movement sleep compared with controls. Other correlates of sleep disturbance included pruritis, scratching movements, higher total serum IgE levels, and allergic sensitization to dust mite and staphylococcal enterotoxins that may be seen or begin to develop during infancy in children with atopy. Lower nocturnal melatonin secretion, although not specifically measured in infants, in the patients with atopic dermatitis was also significantly associated with sleep disturbance.[149]
A study of twin infants in the UK, although of low power, seems to demonstrate a significant involvement of genes and shared family environment on infant sleep behaviors.[150] More research regarding specific environmental determinants may potentially identify specific targets, allowing interventions that would improve infant sleep quality.[150]
Circumcision is most commonly performed in the newborn period, for primarily cosmetic and traditional or cultural reasons. About 85% of males in the US are circumcised at some point during life.[151][152][153] The merits of circumcision remain debated, with most of the non-US world’s male population remaining uncircumcised.[154] Medical benefits include reduced risk of infantile urinary tract infections, estimated at about 23% in uncircumcised children; paraphimosis; phimosis; and balanoposthitis.[155] Neonatal circumcision is also associated with a decreased incidence of penile cancer, a benefit not found when circumcision is performed later in life.[151][152][153][154] There is also evidence of decreased risk of human papillomavirus and HIV infections.[151][156] With the exception of the relative reduction in risk of urinary tract infection, most of the additional medical benefits of circumcision likely may be accomplished without circumcision if access to clean water and proper penile hygiene are achieved. Appropriate penile hygiene will likely eliminate almost all risk of foreskin-related medical problems requiring circumcision. In addition, proper hygiene and clean water access has been shown, in the uncircumcised population, to reduce the risk of penile squamous cell carcinoma.[154]
The primary contraindication to newborn circumcision is bleeding diathesis. Additional reasons to defer infant circumcision are hypospadias, chordee without hypospadias, microphallus, or hidden penis.[153][154]
Circumcision in the newborn may be performed by the infant's pediatrician, if experienced, with either the Gomco or Mogen clamp or the Plastibell device. All of these are low-risk methods and do not typically entail a need for sutures or cautery. A limited comparison of Gomco versus Mogen clamp device for neonatal circumcision found the Mogen clamp to be associated with less neonatal pain (although concurrent use of anesthesia was not addressed in the study) and require a shorter duration of procedure than the Gomco. Both methods were deemed satisfactory to mothers and pediatricians at short-term follow-up.[157]
Complications of properly performed circumcision are infrequent and minor but may potentially have serious sequelae. The most common complication is bleeding, which occurs in about 1% of patients.[158] Most episodes are minor and controlled with direct wound pressure. Gauze, thrombin, topical epinephrine, electrocautery, and sutures may possibly be necessary. Rarely bleeding is of sufficient severity to require transfusion. Infection is second most frequent, with a prevalence of 0.2% to 0.4%.[158] Almost all infections are minor and respond to local wound care and/or antibiotics. Very rarely, infections progress to necrotizing fasciitis or sepsis. Of the long-term complications, recurrent phimosis is probably the most common, but it is difficult to quantify the actual incidence as the majority of revisions performed are for purely cosmetic reasons. Recurrent phimosis often manifests as a concealed penis or a penis that does not appear circumcised due to inadequate circumcision. In extreme cases, the scar formed by inadequate circumcision prevents any exposure of the glans penis and could place the patient at risk for infection. During the healing phase after neonatal circumcision, a skin bridge, thought to be caused by minor injury to the glans at the time of circumcision with resulting fusion to the circumcision wound, may form. Rarely, these skin bridges can result in tethering of the erect penis and pain or penile curvature. If treatment is necessary, it is usually accomplished by simple surgical division in the office with topical anesthetic such as EMLA. Implantation of smegma in the circumcision wound or surgical rolling in of epidermis at the time of circumcision may result in development of inclusion cysts, which may require surgical excision should they become large or infected. A rare and potentially severe complication of circumcision is urethrocutaneous fistula resulting from urethral injury. Typically, this occurs as a result of overzealous treatment of bleeding in the area of the frenulum and will require formal urethral repair 6 to 9 months after the injury. Occurring in 8% to 31% of circumcised boys and having a peak incidence when the child is still in diapers, meatitis likely results from lack of protection to the glans by the prepuce and injury to the urethral meatus by ammonia produced by bacterial action within the urine-soaked diaper. A rare but severe potential complication that may occur during circumcision is accidental traumatic penile amputation, which requires immediate microreplantation of the penis. A variation of this injury is partial amputation of the glans, most commonly occurring with the use of the Mogen clamp device.[154]
Circumcision is a painful procedure, even in newborns, and requires some form of anesthesia or analgesia. Although sucrose is often used for painful procedures in newborns, because it does not reduce pain from intramuscular injection or heel punctures, it would not be acceptable as the sole pain-control intervention.[152][158][159] Opioids may not be effective against injury-induced acute pain, and they may have harmful adverse effects and possibly alter brain development.[160] Local anesthesia, such as topical anesthetic creams, or dorsal penile nerve block is as effective as general and dissociative (e.g., ketamine-induced) anesthesia in preventing a cytokine response associated with procedural pain.[161] These have become the standard of care during neonatal circumcision and the American Academy of Pediatrics recommends that adequate analgesia should be provided whenever a newborn circumcision is performed.[151][158]
Use of this content is subject to our disclaimer