The overall mortality and morbidity associated with cavernous sinus thrombosis (CST) continues to be high.[13]Southwick FS, Richardson EP Jr, Swartz MN. Septic thrombosis of the dural venous sinuses. Medicine. 1986 Mar;65(2):82-106.
http://www.ncbi.nlm.nih.gov/pubmed/3512953?tool=bestpractice.com
Consequently, institution of intensive treatment at the earliest suspicion of disease should be emphasised. Antibiotics are the mainstay of CST therapy. Anticoagulation, corticosteroids, and surgery are adjunctive treatment in appropriately selected patients. Because it is often difficult to distinguish between septic and non-septic causes of CST, the initial management is the same. Only when a septic aetiology is ruled out definitively can antibiotics be withdrawn. In practice, therefore, the treatments are the same for both aseptic and septic disease.[Figure caption and citation for the preceding image starts]: Treatment of aseptic cavernous sinus thrombosis (CST)From the collection of Dr Jayant Pinto, University of Chicago [Citation ends].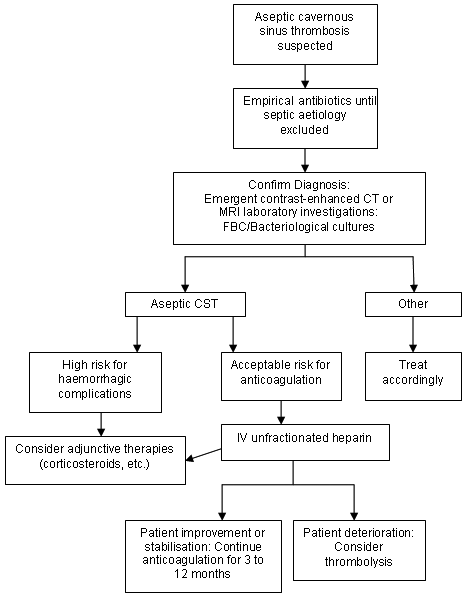 [Figure caption and citation for the preceding image starts]: Treatment of septic cavernous sinus thrombosis (CST)From the collection of Dr Jayant Pinto, University of Chicago [Citation ends].
[Figure caption and citation for the preceding image starts]: Treatment of septic cavernous sinus thrombosis (CST)From the collection of Dr Jayant Pinto, University of Chicago [Citation ends].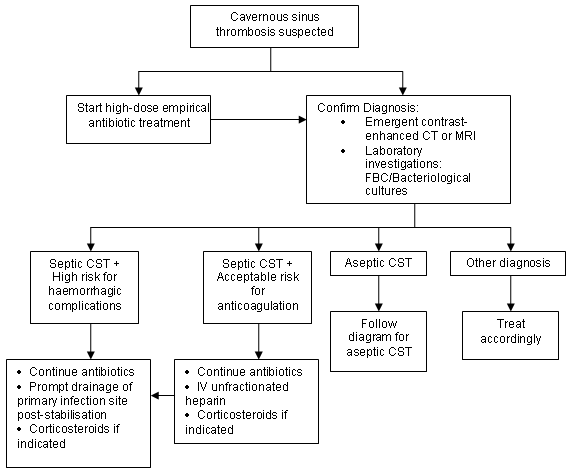
Initial therapy in all patients
Based on case reports and expert opinion, antibiotics are recommended as the mainstay of therapy. They have the greatest impact on the prognosis of septic CST.[2]Yarington CT, Jr. Cavernous sinus thrombosis revisited. Proc R Soc Med. 1977 Jul;70(7):456-9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1543142
http://www.ncbi.nlm.nih.gov/pubmed/331338?tool=bestpractice.com
High-dose intravenous antibiotics should be instituted at the earliest suspicion of this diagnosis.[2]Yarington CT, Jr. Cavernous sinus thrombosis revisited. Proc R Soc Med. 1977 Jul;70(7):456-9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1543142
http://www.ncbi.nlm.nih.gov/pubmed/331338?tool=bestpractice.com
Appropriate selection of empiric antibiotic regimens should be directed at the probable organisms implicated as the primary source of infection. It is necessary to take into account possible complications, such as brain or orbital abscesses, meningitis, or subdural empyema.[1]Ebright JR, Pace MT, Niazi AF. Septic thrombosis of the cavernous sinuses. Arch Intern Med. 2001 Dec 10-24;161(22):2671-6.
http://archinte.ama-assn.org/cgi/content/full/161/22/2671
http://www.ncbi.nlm.nih.gov/pubmed/11732931?tool=bestpractice.com
[48]Bhatia K, Jones NS. Septic cavernous sinus thrombosis secondary to sinusitis: are anticoagulants indicated? A review of the literature. J Laryngol Otol. 2002 Sep;116(9):667-76.
http://www.ncbi.nlm.nih.gov/pubmed/12437798?tool=bestpractice.com
Staphylococcus aureus is the most common pathogen, identified in approximately 70% of cases and is the pathogen implicated in nearly all cases of facial infections.[1]Ebright JR, Pace MT, Niazi AF. Septic thrombosis of the cavernous sinuses. Arch Intern Med. 2001 Dec 10-24;161(22):2671-6.
http://archinte.ama-assn.org/cgi/content/full/161/22/2671
http://www.ncbi.nlm.nih.gov/pubmed/11732931?tool=bestpractice.com
Bacteria associated with sinusitis include Fusobacterium necrophorum and Streptococci (including S pneumoniae, S milleri, and S viridans group).[11]van der Poel NA, Mourits MP, de Win MML, et al. Prognosis of septic cavernous sinus thrombosis remarkably improved: a case series of 12 patients and literature review. Eur Arch Otorhinolaryngol. 2018 Sep;275(9):2387-95.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6096574
http://www.ncbi.nlm.nih.gov/pubmed/29998385?tool=bestpractice.com
Anaerobes are found occasionally, especially with sinus, dental, or tonsillar infections. Rarely, fungal infection from Aspergillus fumigatus or mucormycosis have been implicated in CST.[74]Estrem SA, Tully R, Davis WE. Rhinocerebral mucormycosis: computed tomographic imaging of cavernous sinus thrombosis. Ann Otol Rhinol Laryngol. 1990 Feb;99(2 Pt 1):160-1.
http://www.ncbi.nlm.nih.gov/pubmed/2301872?tool=bestpractice.com
[75]Dooley DP, Hollsten DA, Grimes SR, et al. Indolent orbital apex syndrome caused by occult mucormycosis. J Clin Neuroophthalmol. 1992 Dec;12(4):245-9.
http://www.ncbi.nlm.nih.gov/pubmed/1287049?tool=bestpractice.com
For empirical antibiotic therapy options, based on expert opinion, may include amoxicillin/clavulanate plus gentamicin, a third-generation cephalosporin, a fluoroquinolone, and the addition of metronidazole if brain abscess or dental or sinus infection is suspected.[6]Weerasinghe D, Lueck CJ. Septic cavernous sinus thrombosis: case report and review of the literature. Neuroophthalmology. 2016 Dec;40(6):263-76.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5120738
http://www.ncbi.nlm.nih.gov/pubmed/27928417?tool=bestpractice.com
[76]Sonneville R, Ruimy R, Benzonana N, et al. An update on bacterial brain abscess in immunocompetent patients. Clin Microbiol Infect. 2017 Sep;23(9):614-20.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(17)30259-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/28501669?tool=bestpractice.com
[77]Berdai AM, Shimi A, Khatouf M. Cavernous sinus thrombophlebitis complicating sinusitis. Am J Case Rep. 2013;14:99-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3700470
http://www.ncbi.nlm.nih.gov/pubmed/23826444?tool=bestpractice.com
[78]Aloua R, Kerdoud O, Slimani F. Cavernous sinus thrombosis related to orbital cellulitis serious complication to prevent: a case report and literature review. Ann Med Surg (Lond). 2021 Feb;62:179-81.
https://www.sciencedirect.com/science/article/pii/S2049080121000388?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/33532066?tool=bestpractice.com
Consider adding vancomycin or linezolid if methicillin-resistant S aureus infection is suspected.[79]Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011 Feb 1;52(3):e18-55.
https://academic.oup.com/cid/article/52/3/e18/306145
http://www.ncbi.nlm.nih.gov/pubmed/21208910?tool=bestpractice.com
Consult your local guidelines or infectious disease consultant for more information as this is a very specialised area with little evidence available to guide treatment decisions.
Antifungal therapy has been advocated only in cases of biopsy-confirmed invasive fungal infection. However, in at-risk patients, such as patients with immunocompromise or poorly controlled diabetes, antifungal treatment should be considered as fungi may cause devastating neurological complications beyond cerebral venous thrombosis (CVT).[80]Korathanakhun P, Petpichetchian W, Sathirapanya P, et al. Cerebral venous thrombosis: comparing characteristics of infective and non-infective aetiologies: a 12-year retrospective study. Postgrad Med J. 2015 Dec;91(1082):670-4.
http://www.ncbi.nlm.nih.gov/pubmed/26499451?tool=bestpractice.com
As soon as the laboratory has reported sensitivities, empiric antibiotics can be switched to specific antibiotic therapy.
High doses of intravenous antibiotics are required because thrombus may limit penetration of antibiotics. Bacteria, sequestered within the thrombus, may not be killed until the dural sinuses have started to re-canalise. Antibiotics also need to be administered over an extended period, for at least 3-4 weeks.[39]Dolapsakis C, Kranidioti E, Katsila S, et al. Cavernous sinus thrombosis due to ipsilateral sphenoid sinusitis. BMJ Case Rep. 2019 Jan 29;12(1):e227302.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6352844
http://www.ncbi.nlm.nih.gov/pubmed/30700458?tool=bestpractice.com
This aims to insure complete sterilisation and prevent relapses.
Concurrent supportive therapy is necessary alongside antibiotic treatment, and includes resuscitation, oxygen support, and local eye care.[5]Caranfa JT, Yoon MK. Septic cavernous sinus thrombosis: a review. Surv Ophthalmol. 2021 Nov-Dec;66(6):1021-30.
http://www.ncbi.nlm.nih.gov/pubmed/33831391?tool=bestpractice.com
Adjunctive therapy: anticoagulation
Anticoagulation is the standard of care in the management of aseptic CST; however, considerable controversy exists concerning the efficacy of anticoagulation in the treatment of septic CST.[39]Dolapsakis C, Kranidioti E, Katsila S, et al. Cavernous sinus thrombosis due to ipsilateral sphenoid sinusitis. BMJ Case Rep. 2019 Jan 29;12(1):e227302.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6352844
http://www.ncbi.nlm.nih.gov/pubmed/30700458?tool=bestpractice.com
Prospective trials to establish any benefit from anticoagulation have never been (and are unlikely to be) performed owing to the rarity of the condition.[39]Dolapsakis C, Kranidioti E, Katsila S, et al. Cavernous sinus thrombosis due to ipsilateral sphenoid sinusitis. BMJ Case Rep. 2019 Jan 29;12(1):e227302.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6352844
http://www.ncbi.nlm.nih.gov/pubmed/30700458?tool=bestpractice.com
Anticoagulation carries the risk of haemorrhage, especially in patients with concomitant complications (e.g., cortical venous infarction, necrosis of intra-cavernous portions of the carotid artery, and cerebral or intra-orbital haemorrhages).[48]Bhatia K, Jones NS. Septic cavernous sinus thrombosis secondary to sinusitis: are anticoagulants indicated? A review of the literature. J Laryngol Otol. 2002 Sep;116(9):667-76.
http://www.ncbi.nlm.nih.gov/pubmed/12437798?tool=bestpractice.com
However, there is some evidence that the use of anticoagulation prevents propagation and contributes to re-canalisation of the thrombus. These are potentially beneficial effects, partly because the thrombus itself can harbour bacteria and sustain their growth.[2]Yarington CT, Jr. Cavernous sinus thrombosis revisited. Proc R Soc Med. 1977 Jul;70(7):456-9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1543142
http://www.ncbi.nlm.nih.gov/pubmed/331338?tool=bestpractice.com
Two retrospective reviews examining the use of anticoagulation for septic CST produced varying results.[13]Southwick FS, Richardson EP Jr, Swartz MN. Septic thrombosis of the dural venous sinuses. Medicine. 1986 Mar;65(2):82-106.
http://www.ncbi.nlm.nih.gov/pubmed/3512953?tool=bestpractice.com
[19]Levine SR, Twyman RE, Gilman S. The role of anticoagulation in cavernous sinus thrombosis. Neurology. 1988 Apr;38(4):517-22.
http://www.ncbi.nlm.nih.gov/pubmed/3281056?tool=bestpractice.com
One subsequent individual patient data meta-analysis (110 patients from 72 articles), published in 2025, concluded that the use of anticoagulation was associated with reduced mortality in patients with septic CST.[81]Akarapas C, Wiwatkunupakarn N, Sithirungson S, et al. Anticoagulation for cavernous sinus thrombosis: a systematic review and individual patient data meta-analysis. Eur Arch Otorhinolaryngol. 2025 Mar;282(3):1127-34.
http://www.ncbi.nlm.nih.gov/pubmed/39312001?tool=bestpractice.com
Based on limited observation, anticoagulation may be beneficial in patients with septic CST after exclusion of haemorrhagic complications by CT scan.[2]Yarington CT, Jr. Cavernous sinus thrombosis revisited. Proc R Soc Med. 1977 Jul;70(7):456-9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1543142
http://www.ncbi.nlm.nih.gov/pubmed/331338?tool=bestpractice.com
[13]Southwick FS, Richardson EP Jr, Swartz MN. Septic thrombosis of the dural venous sinuses. Medicine. 1986 Mar;65(2):82-106.
http://www.ncbi.nlm.nih.gov/pubmed/3512953?tool=bestpractice.com
[19]Levine SR, Twyman RE, Gilman S. The role of anticoagulation in cavernous sinus thrombosis. Neurology. 1988 Apr;38(4):517-22.
http://www.ncbi.nlm.nih.gov/pubmed/3281056?tool=bestpractice.com
Anticoagulation is thought by some to be dangerous in patients with bilateral CST and/or concurrent intracranial haemorrhage.
The types and protocols for anticoagulation have varied considerably in research protocols. Intravenous and intramuscular unfractionated heparin, subcutaneous low molecular-weight heparin, and oral anticoagulation have all been used. However, the use of a rapidly reversible agent, such as intravenous unfractionated heparin has been advocated in the early stages of disease, followed by conversion to longer-acting agents, such as warfarin, when the patient's condition has stabilised.[48]Bhatia K, Jones NS. Septic cavernous sinus thrombosis secondary to sinusitis: are anticoagulants indicated? A review of the literature. J Laryngol Otol. 2002 Sep;116(9):667-76.
http://www.ncbi.nlm.nih.gov/pubmed/12437798?tool=bestpractice.com
Limited evidence in patients with CVT suggests that direct oral anticoagulants (DOACs) and warfarin may have comparable efficacy and safety.[82]Yaghi S, Saldanha IJ, Misquith C, et al. Direct oral anticoagulants versus vitamin K antagonists in cerebral venous thrombosis: a systematic review and meta-analysis. Stroke. 2022 Oct;53(10):3014-24.
https://www.ahajournals.org/doi/10.1161/STROKEAHA.122.039579?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/35938419?tool=bestpractice.com
[83]Bose G, Graveline J, Yogendrakumar V, et al. Direct oral anticoagulants in treatment of cerebral venous thrombosis: a systematic review. BMJ Open. 2021 Feb 16;11(2):e040212.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7888326
http://www.ncbi.nlm.nih.gov/pubmed/33593766?tool=bestpractice.com
Newer anticoagulants, including direct thrombin inhibitors and factor Xa inhibitors, offer many advantages over heparin, including a more predictable anticoagulant effect and an absence of induction of immune-mediated heparin-induced thrombocytopenia (HIT).[84]Direct Thrombin Inhibitor Trialists' Collaborative Group. Direct thrombin inhibitors in acute coronary syndromes: principal results of a meta-analysis based on individual patients' data. Lancet. 2002 Jan 26;359(9303):294-302.
http://www.ncbi.nlm.nih.gov/pubmed/11830196?tool=bestpractice.com
However, there is a lack of reported cases of CST or other forms of dural sinus thrombosis that have been treated with these agents. The use of alternative anticoagulants (e.g., argatroban) can be considered in patients with HIT or those at risk of HIT.
The duration of anticoagulation has not been determined and varies in reports from a few weeks to several months.[5]Caranfa JT, Yoon MK. Septic cavernous sinus thrombosis: a review. Surv Ophthalmol. 2021 Nov-Dec;66(6):1021-30.
http://www.ncbi.nlm.nih.gov/pubmed/33831391?tool=bestpractice.com
[6]Weerasinghe D, Lueck CJ. Septic cavernous sinus thrombosis: case report and review of the literature. Neuroophthalmology. 2016 Dec;40(6):263-76.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5120738
http://www.ncbi.nlm.nih.gov/pubmed/27928417?tool=bestpractice.com
Some authors have suggested that anticoagulation should be continued until clinical or radiological evidence of complete resolution is present, or until there is significant improvement of the infection and thrombus.
Patients commenced on anticoagulants are usually still in an unstable clinical condition and are therefore not candidates for surgical management. However, if the patient's condition stabilises and surgical management is indicated, rapidly reversible anticoagulants can be discontinued to allow surgery.
If a patient is considered suitable for anticoagulation but deteriorates despite this therapy, they may be considered for endovascular therapy.[85]Liebetrau M, Mayer TE, Bruning R, et al. Intra-arterial thrombolysis of complete deep cerebral venous thrombosis. Neurology. 2004 Dec 28;63(12):2444-5.
http://www.ncbi.nlm.nih.gov/pubmed/15623729?tool=bestpractice.com
[86]Canhão P, Falcão F, Ferro JM. Thrombolytics for cerebral sinus thrombosis: a systematic review. Cerebrovasc Dis. 2003;15(3):159-66.
http://www.ncbi.nlm.nih.gov/pubmed/12646773?tool=bestpractice.com
[87]Saposnik G, Barinagarrementeria F, Brown RD Jr, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 Apr;42(4):1158-92.
https://www.ahajournals.org/doi/10.1161/STR.0b013e31820a8364?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/21293023?tool=bestpractice.com
Although endovascular treatment is increasingly being used to treat patients with CVT, this treatment is not routinely recommended in all patients.[88]Coutinho JM, Zuurbier SM, Bousser MG, et al. Effect of endovascular treatment with medical management vs standard care on severe cerebral venous thrombosis: the TO-ACT randomized clinical trial. JAMA Neurol. 2020 Aug 1;77(8):966-73.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7235912
http://www.ncbi.nlm.nih.gov/pubmed/32421159?tool=bestpractice.com
For patients with CST this therapy is usually reserved for progressive, aseptic CST and carries with it the risks of intracranial haemorrhage, stroke, and the inability to recanalise. It does not preclude corticosteroids.
Adjunctive therapy: corticosteroids
The role of corticosteroids is controversial in many cases of CST. They are potentially harmful because of their immunosuppressive effects. However, corticosteroids are absolutely indicated in cases of pituitary insufficiency. Corticosteroid use may have a critical role in patients with Addisonian crisis secondary to ischaemia or necrosis of the pituitary that complicates CST.[89]Silver HS, Morris LR. Hypopituitarism secondary to cavernous sinus thrombosis. South Med J. 1983 May;76(5):642-6.
http://www.ncbi.nlm.nih.gov/pubmed/6302919?tool=bestpractice.com
[90]Sahjpaul RL, Lee DH. Infratentorial subdural empyema, pituitary abscess, and septic cavernous sinus thrombophlebitis secondary to paranasal sinusitis: case report. Neurosurgery. 1999 Apr;44(4):864-6; discussion 866-8.
http://www.ncbi.nlm.nih.gov/pubmed/10201313?tool=bestpractice.com
Although there would seem to be only empiric support for their anti-inflammatory properties, with a real fear of progression to generalised sepsis, corticosteroids may also be beneficial for:[5]Caranfa JT, Yoon MK. Septic cavernous sinus thrombosis: a review. Surv Ophthalmol. 2021 Nov-Dec;66(6):1021-30.
http://www.ncbi.nlm.nih.gov/pubmed/33831391?tool=bestpractice.com
There are only a few anecdotal reports concerning the use of corticosteroids in CST in general and their efficacy has not been proved by these reports. In the studies in which the use of corticosteroids has been reported, other treatments have been used concurrently.[13]Southwick FS, Richardson EP Jr, Swartz MN. Septic thrombosis of the dural venous sinuses. Medicine. 1986 Mar;65(2):82-106.
http://www.ncbi.nlm.nih.gov/pubmed/3512953?tool=bestpractice.com
[37]Gallagher RM, Gross CW, Phillips CD. Suppurative intracranial complications of sinusitis. Laryngoscope. 1998 Nov;108(11 Pt 1):1635-42.
http://www.ncbi.nlm.nih.gov/pubmed/9818818?tool=bestpractice.com
[91]Clifford-Jones RE, Ellis CJ, Stevens JM, et al. Cavernous sinus thrombosis. J Neurol Neurosurg Psychiaty. 1982 Dec;45(12):1092-7.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC491689
http://www.ncbi.nlm.nih.gov/pubmed/7161604?tool=bestpractice.com
[92]Igarashi H, Igarashi S, Fujio N, et al. Magnetic resonance imaging in the early diagnosis of cavernous sinus thrombosis. Ophthalmologica. 1995;209(5):292-6.
http://www.ncbi.nlm.nih.gov/pubmed/8570157?tool=bestpractice.com
In one case, reported in 1962, cranial nerve dysfunction and orbital oedema failed to improve after 37 days of antibiotic and anticoagulant therapy but regressed dramatically 2 days after the addition of corticosteroid therapy, with eventual complete resolution in eye signs and symptoms.[93]Solomon OD, Moses L, Volk M. Steroid therapy in cavernous sinus thrombosis. Am J Ophthalmol. 1962 Dec;54:1122-4.
http://www.ncbi.nlm.nih.gov/pubmed/13978082?tool=bestpractice.com
Surgical drainage post-stabilisation
Finally, as soon as the patient's condition permits, prompt drainage of the primary site of infection (such as the para-nasal sinusitis, dental abscess) or other concurrent closed-space infection is advisable.[7]DiNubile MJ. Septic thrombosis of the cavernous sinuses. Arch Neurol. 1988 May;45(5):567-72.
http://www.ncbi.nlm.nih.gov/pubmed/3282499?tool=bestpractice.com
[13]Southwick FS, Richardson EP Jr, Swartz MN. Septic thrombosis of the dural venous sinuses. Medicine. 1986 Mar;65(2):82-106.
http://www.ncbi.nlm.nih.gov/pubmed/3512953?tool=bestpractice.com
[94]Mahapatra AK. Brain abscess-an unusual complication of cavernous sinus thrombosis. A case report. Clin Neurol Neurosurg. 1988;90(3):241-3.
http://www.ncbi.nlm.nih.gov/pubmed/3197350?tool=bestpractice.com
Surgical drainage of the cavernous sinus is almost never performed.[1]Ebright JR, Pace MT, Niazi AF. Septic thrombosis of the cavernous sinuses. Arch Intern Med. 2001 Dec 10-24;161(22):2671-6.
http://archinte.ama-assn.org/cgi/content/full/161/22/2671
http://www.ncbi.nlm.nih.gov/pubmed/11732931?tool=bestpractice.com
In sinogenic CST, surgical drainage of the sinuses for all cases has been advocated.[10]Wang YH, Chen PY, Ting PJ, et al. A review of eight cases of cavernous sinus thrombosis secondary to sphenoid sinusitis, including a 12-year-old girl at the present department. Infect Dis (Lond). 2017 Sep;49(9):641-6.
http://www.ncbi.nlm.nih.gov/pubmed/28535728?tool=bestpractice.com
[11]van der Poel NA, Mourits MP, de Win MML, et al. Prognosis of septic cavernous sinus thrombosis remarkably improved: a case series of 12 patients and literature review. Eur Arch Otorhinolaryngol. 2018 Sep;275(9):2387-95.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6096574
http://www.ncbi.nlm.nih.gov/pubmed/29998385?tool=bestpractice.com
[95]Dolan RW, Chowdhury K. Diagnosis and treatment of intracranial complications of paranasal sinus infections. J Oral Maxillofac Surg. 1995 Sep;53(9):1080-7.
http://www.ncbi.nlm.nih.gov/pubmed/7643279?tool=bestpractice.com
Different operations have been performed to decompress the sinuses, including trans-septal sphenoidectomy, endoscopic sphenoidectomy and ethmoidectomy and external fronto-ethmoidal-sphenoidectomy. In cases of otogenic CST, mastoidectomy has been performed, with decompression of sigmoid sinus thrombophlebitis.[40]Doyle KJ, Jackler RK. Otogenic cavernous sinus thrombosis. Otolaryngol Head Neck Surg. 1991 Jun;104(6):873-7.
http://www.ncbi.nlm.nih.gov/pubmed/1908984?tool=bestpractice.com
 [Figure caption and citation for the preceding image starts]: Treatment of septic cavernous sinus thrombosis (CST)From the collection of Dr Jayant Pinto, University of Chicago [Citation ends].
[Figure caption and citation for the preceding image starts]: Treatment of septic cavernous sinus thrombosis (CST)From the collection of Dr Jayant Pinto, University of Chicago [Citation ends].