Approach
The majority of patients require surgery to ligate the fistula and establish oesophageal continuity. The condition is not compatible with life as the patient cannot eat and is at significant risk for aspiration. Patients with pure atresia and no fistula also require surgery but can be managed either by direct gastric feeds or intravenous hyperalimentation with a delayed repair of the oesophagus. Before surgery, the patient is stabilised by placement of a nasogastric tube to decompress the upper blind pouch.[17]
A clinically challenging subset of oesophageal atresia (OA) is known as long-gap OA which has been defined by the International Network of Esophageal Atresia as types that are technically difficult to repair.[23] Gap lengths can vary, but preservation and use of the native esophagus should be the driving principle. However, when necessary, a number of visceral replacements are feasible.[24]
Gross classification: the surgery of infancy and childhood, 1954
Type A: pure atresia (4% to 7%)
Type B: proximal fistula with distal atresia (1%)
Type C: proximal atresia with distal fistula (85% to 90%)
Type D: proximal and distal fistula (3%)
Type E: H-type fistula (2% to 3%); tracheo-oesophageal fistula (TOF) without OA.[1]
[Figure caption and citation for the preceding image starts]: Types of tracheo-oesophageal fistula/oesophageal atresia based on Gross classificationCreated by the BMJ Knowledge Centre [Citation ends].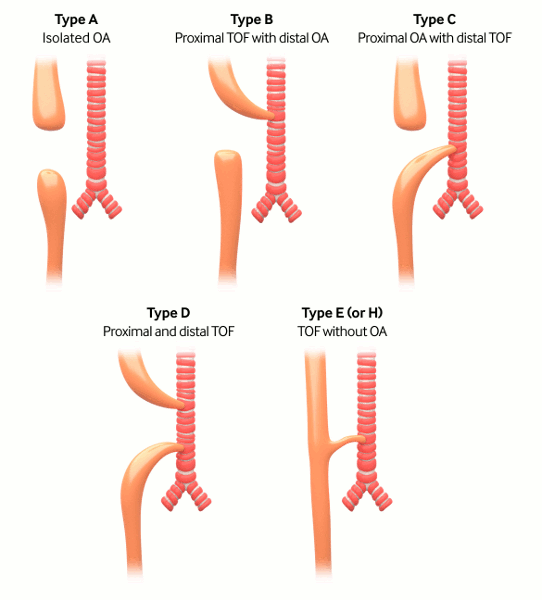
Type A
Surgical oesophageal repair at birth is often challenging. However, without a connection to the trachea, these patients are less susceptible to acute respiratory distress.
Patients may be managed with a nasogastric tube in the upper oesophageal pouch, providing continuous suction. Within the first few days of life, a gastrostomy tube is placed to allow for enteral feeds and the stomach contrast study to identify the length of the lower pouch. If the length between the proximal and distal oesophageal segments is 4 vertebral bodies or longer, then the patient is allowed to grow prior to attempting a repair.[25] Growth is monitored by using contrast radiographs, also known as a 'gap study'. Typically, a probe is inserted through the gastrostomy site into the distal pouch and the nasogastric tube is advanced to the end of the proximal pouch to define the gap. If the gap between the proximal and distal segments of the oesophagus is less than 2 vertebral bodies, primary oesophageal repair can be performed immediately or at around 2 to 3 months of age. Originally, repair was performed via a right thoracotomy, although this procedure is now performed thoracoscopically.
In cases of a very long gap, various techniques have been designed to stretch or increase the oesophageal length.
In some cases of a failed oesophageal repair or an extremely long gap, oesophageal replacement surgery is planned.
Type B
These patients cannot eat and are at risk for repeated aspiration of oral secretions due to a proximal fistula. The patients can be managed initially by a suction catheter in the upper oesophageal pouch which should limit secretions going into the trachea. Surgery should take place in the first 24 to 48 hours of life to ligate and divide the fistula and establish oesophageal continuity.
Type C
The first-line treatment is with surgical correction, aimed at dividing the TOF to prevent lung aspiration. Then, anastomosis of the 2 oesophageal ends is completed, in order to establish continuity of the oesophagus. OA/TOF repair was previously performed via a right thoracotomy but is now performed by many paediatric surgeons using a thoracoscopic approach. Meta-analysis indicates that outcomes for either method are similar, and although the thoracoscopic procedure is associated with a longer operative time and contraindications such as severe haemodynamic instability, it may also reduce the first oral feeding time and length of hospital stay.[17][26][27] The operation should be performed within the first 24 hours of life to prevent complications of aspiration and abdominal distension. A 2021 consortia demonstrated that the elimination of transanastomotic tubes was associated with decreased stricture rates without delaying time to initiation of per ora feeds.[28] In unstable premature infants with type C OA/TOF, staged approaches such as ligation of fistula alone, or temporary occlusion of the gastroesophageal junction may be indicated. Some advocate for performing a posterior tracheopexy on all infants at the time of TOF repair whereas others perform it more selectively.[29] Posterior tracheopexy and rotational oesophagoplasty are good techniques to use when planning surgery for a recurrent TOF.[30]
Type D
A suction catheter should be placed prior to surgery to decompress the upper pouch and limit the secretions entering the trachea. Surgical management includes division of the fistulas and an anastomosis of the proximal and distal oesophageal pouches.
Type E
These patients often present in late childhood or in early adulthood with evidence of choking, gagging or recurrent aspiration. Once diagnosed, the patient should be kept nothing by mouth until the fistula is divided. Through a right neck incision at the level of the thoracic inlet, fistula repair is performed. The fistula can also be reached thoracoscopically through the right chest.
Use of this content is subject to our disclaimer