History and exam
Key diagnostic factors
common
presence of risk factors
Risk factors include high exposure to sand flies, poor disease awareness, proximity to infected patients, and immunosuppression.
previous stay in endemic area
Feature of cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL).
A complete history of travel is necessary to identify previous exposure in CL- and VL-endemic areas: parts of Latin America, Mediterranean basin, Middle East, Central Asia, sub-Saharan Africa (in particular East Africa), northern India, southern Nepal, or north-west Bangladesh.
Incubation period can be variable and depends on parasite species.[1][2]
[Figure caption and citation for the preceding image starts]: Status of the endemicity of cutaneous leishmaniasis worldwide, 2016Image courtesy of the World Health Organization [Citation ends].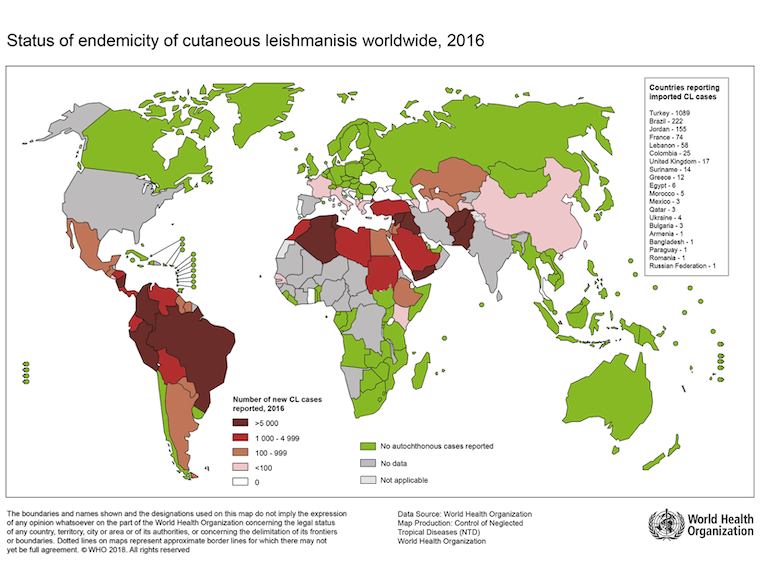 [Figure caption and citation for the preceding image starts]: Status of endemicity of visceral leishmaniasis (VL) worldwide, 2020Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO (https://creativecommons.org/licenses/by-nc-sa/3.0/igo/) [Citation ends].
[Figure caption and citation for the preceding image starts]: Status of endemicity of visceral leishmaniasis (VL) worldwide, 2020Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO (https://creativecommons.org/licenses/by-nc-sa/3.0/igo/) [Citation ends].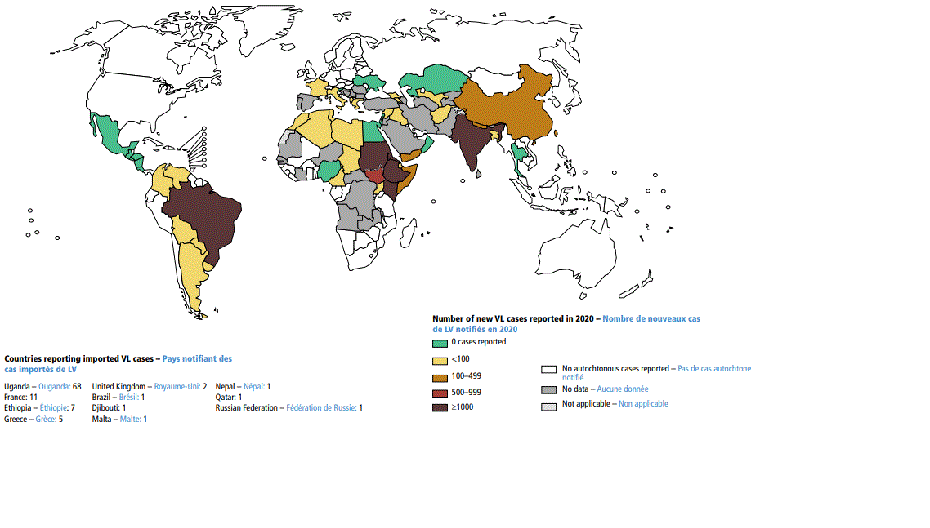
immunosuppression
Feature of visceral leishmaniasis.
Cell-mediated immunosuppression.
prolonged fever
Feature of visceral leishmaniasis.
Prolonged (weeks or months) and may be intermittent.[2] Classically described as double quotidian (two episodes of fever occur daily).
weight loss
Feature of visceral leishmaniasis.
Weight loss is due to anorexia and persistent inflammatory state.
Concomitant infections (e.g., HIV, tuberculosis, diarrhoea) may be aggravating factors.[2]
ulcerative skin lesions
Characteristic clinical sign of cutaneous leishmaniasis (CL).
Sometimes occurs in visceral leishmaniasis (e.g., immunocompromised patients).
Single or multiple lesions, often painless and persistent. Lesions can vary in appearance, although the classic localised CL lesions are characterised by a volcano-like appearance (i.e., raised, indurated borders with depressed, ulcerative lesion centre) at the bite site.
Tends to affect skin that is readily exposed to sand fly bites (i.e., face, arms, and lower limbs). May develop at distant sites, such as areas of minor trauma (e.g., bee sting, new tattoo, surgical incision), or may be disseminated in immunocompromised hosts.[11] Lesions are occasionally reported on atypical areas (e.g., sexual organs).[9]
An auricular manifestation of CL is a single ulcerative lesion, typically involving the ear pinna (known as ‘chiclero’s’ ulcer in southeast Mexico and Latin America when caused by Leishmania mexicana).[10]
The Old World CL ulcer often has a dry crust covering it.[1][Figure caption and citation for the preceding image starts]: Ulcerative Leishmania braziliensis lesion from a student who travelled to PeruFrom the collection of Dr N. Aronson; used with permission [Citation ends].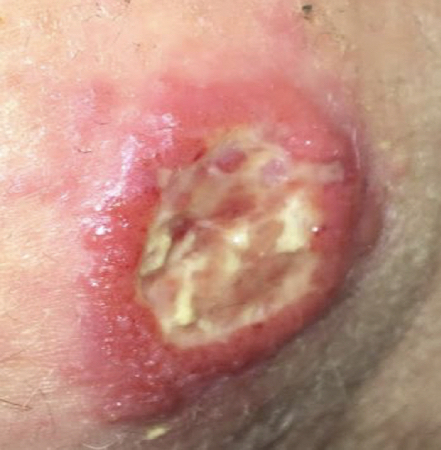 [Figure caption and citation for the preceding image starts]: Ulcerative Leishmania mexicana lesion, pre- and post-treatmentFrom the collection of Dr N. Aronson; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Ulcerative Leishmania mexicana lesion, pre- and post-treatmentFrom the collection of Dr N. Aronson; used with permission [Citation ends].
multiple non-ulcerative skin nodules
Characteristic of diffuse cutaneous leishmaniasis.
destructive mucosal inflammation
Characteristic of mucosal leishmaniasis.[Figure caption and citation for the preceding image starts]: Mucosal leishmaniasis in 2 Brazilian patients. A-D: patient 1 with positron emission tomography/computed tomography (PET/CT) images showing enhancement and subcutaneous thickening adjacent to erosion of the left nasal wing and obliteration of the posterolateral recess (A and B), 3D volume-rendered image of multislice CT data (3D CT) and picture with erosion of the left nasal wing (C and D). F-H: patient 2 with PET/CT images showing preserved glycolytic metabolism of facial structures (E and F), 3D CT with collapse of the nasal pyramids (G), and bone window CT with diffuse thickening of nasal wings and collapse of the nasal pyramid (H)Am J Trop Med Hyg; CC BY-4.0 (https://creativecommons.org/licenses/by/4.0/) [Citation ends].
splenomegaly
Associated with visceral leishmaniasis.
Can be massive and symptomatic.
Palpation of the spleen is usually painless.[2][51][Figure caption and citation for the preceding image starts]: Hepatosplenomegaly in an Ethiopian patient with visceral leishmaniasisImage courtesy of the World Health Organization [Citation ends].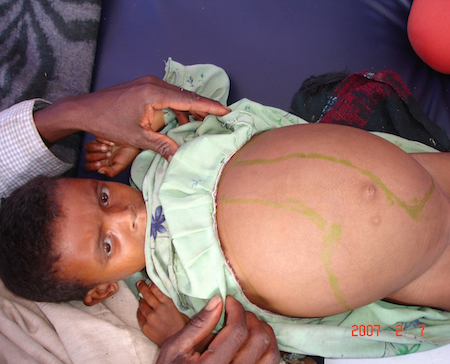
uncommon
skin darkening
Feature of visceral leishmaniasis.
Generally observed by the patient or family.
Described in South Asia (kala-azar).
Other diagnostic factors
common
fatigue
Feature of visceral leishmaniasis.
Pronounced, due to the persistent inflammatory state, weight loss, and anaemia.[2]
cough
Feature of visceral leishmaniasis.
headache
Feature of visceral leishmaniasis.
wasting
Characteristic of visceral leishmaniasis.
enlarged lymph nodes
Commonly associated with cutaneous leishmaniasis (CL); less commonly associated with visceral leishmaniasis (VL).
Enlarged lymph nodes in CL are in the local and regional lymphatic drainage distribution, sometimes leading to a sporotrichoid appearance.
In Sudan, it is common for CL and VL.
Often generalised in VL, if present.
hepatomegaly
Associated with visceral leishmaniasis.
Less massive than splenomegaly.
Liver palpation is usually painless.[2]
uncommon
previous anti-leishmanial treatment
Feature of cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL).
Raises suspicion of relapse in case of recurrent symptoms of CL or VL, or post-kala-azar dermal leishmaniasis if consistent skin signs are present.
epistaxis
Feature of visceral leishmaniasis.
The aetiology of epistaxis is poorly understood.
Thrombocytopenia is likely to be a risk factor.[2]
abdominal pain
Atypical feature found in patients with immunosuppression.
Risk factors
strong
high exposure to sand fly bites
A prolonged stay in zones of intense Leishmania transmission, such as rural areas of Afghanistan, Iraq, the State of Bihar in India, or eastern Sudan, increases risk. Use of personal protective measures including applying diethyltoluamide (DEET) to exposed skin, wearing permethrin-treated clothing that covers skin at night, and sleeping in permethrin-treated bed nets are advised to reduce the risk of sand fly bites. Risk is particularly from dusk to dawn.
Bed net use and spraying of the home with insecticides have been shown to protect against infection and/or disease caused by some sand fly species.[48][49][50]
poverty
Poverty increases the risk of leishmaniasis. Poor housing and sanitary conditions may increase sand fly breeding sites and vector access to humans. Additionally, malnutrition contributes to a poor immune response to the parasite.
proximity to a patient with a history of infection
Only a risk in anthroponotic disease (humans are the major or sole infection reservoir). This applies to Leishmania tropica for cutaneous leishmaniasis and Leishmania donovani for visceral leishmaniasis.
ownership of domestic animals
immunosuppression
Co-infection with HIV, use of immunosuppressive drugs (e.g., post-transplantation, biological modifying agents such as tumour necrosis factor [TNF]-alpha antagonists), severe malnutrition, and immunosuppression associated with malignancy all increase the risk of developing active cutaneous leishmaniasis and visceral leishmaniasis (VL).[5][17][46][47][56][57][58]
CD4 counts <200 cells/microlitre and failure to take antiretroviral therapy (ART) are strong risk factors for VL in HIV-infected patients.
The widespread use of ART had a strong impact in decreasing the number of diagnosed HIV-VL co-infections in Europe; however, co-infection in developing countries continues to be increasingly reported.[17][59]
Use of this content is subject to our disclaimer