Prevention of tetanus is always preferable to management of the clinical tetanus syndrome. See Primary prevention.
The management of clean and tetanus-prone wounds should take into account the patient's immunisation status. Immunosuppressed patients may not be adequately protected and additional boosting and/or tetanus immunoglobulin (TIG) may be required; in the US, immunosuppressed patients should be managed as if they were incompletely immunised.[3]Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018 Apr 27;67(2):1–44.
https://www.cdc.gov/mmwr/volumes/67/rr/rr6702a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/29702631?tool=bestpractice.com
[4]UK Health Security Agency. Tetanus: the green book, chapter 30. Jun 2022 [internet publication].
https://www.gov.uk/government/publications/tetanus-the-green-book-chapter-30
[36]Tiwari TSP, Moro PL, Acosta AM. Tetanus. In: Hall E, Wodi AP, Hamborsky J, et al, eds. Centers for Disease Control and Prevention. Epidemiology and vaccine-preventable diseases. 14th ed. Washington, D.C.: Public Health Foundation; 2021.
https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html
The principles of management for the clinical tetanus syndrome include supportive care, wound debridement, antimicrobials, passive and active immunisation, control of muscle spasms, and management of autonomic dysfunction.[1]Brook I. Current concepts in the management of Clostridium tetani infection. Expert Rev Anti Infect Ther. 2008 Jun;6(3):327-36.
http://www.ncbi.nlm.nih.gov/pubmed/18588497?tool=bestpractice.com
[43]Rodrigo C, Fernando D, Rajapakse S. Pharmacological management of tetanus: an evidence-based review. Crit Care. 2014 Mar 26;18(2):217.
https://ccforum.biomedcentral.com/articles/10.1186/cc13797
http://www.ncbi.nlm.nih.gov/pubmed/25029486?tool=bestpractice.com
[44]Yen LM, Thwaites CL. Tetanus. Lancet. 2019 Apr 20;393(10181):1657-68.
http://www.ncbi.nlm.nih.gov/pubmed/30935736?tool=bestpractice.com
International recommendations on the management of clinical tetanus and tetanus-prone wounds may vary, and local guidelines should be consulted.
Management of clean and minor wounds and tetanus-prone wounds
Tetanus toxoid-containing vaccine and/or TIG may be used depending on the patient’s immunisation history and risk assessment of the wound.
Wounds or burns that are considered to be tetanus prone include the following:[3]Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018 Apr 27;67(2):1–44.
https://www.cdc.gov/mmwr/volumes/67/rr/rr6702a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/29702631?tool=bestpractice.com
[4]UK Health Security Agency. Tetanus: the green book, chapter 30. Jun 2022 [internet publication].
https://www.gov.uk/government/publications/tetanus-the-green-book-chapter-30
Tetanus toxoid is only available in combination with other antigens such as diphtheria and pertussis. The following vaccines are recommended for active vaccination in patients with tetanus-prone wounds: diphtheria/tetanus/acellular pertussis vaccine (DTaP); tetanus/diphtheria vaccine (Td for children ≥7 years of age and adults; or DT for children up to 7 years of age); and tetanus/low-dose diphtheria/acellular pertussis vaccine (Tdap). DTaP is recommended for children aged <7 years. DT is used when the pertussis vaccine component is contraindicated. Tdap can be given if the person is 11 years of age or older and has not yet received Tdap.[3]Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018 Apr 27;67(2):1–44.
https://www.cdc.gov/mmwr/volumes/67/rr/rr6702a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/29702631?tool=bestpractice.com
[34]Havers FP, Moro PL, Hunter P, et al. Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: updated recommendations of the Advisory Committee on Immunization Practices - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020 Jan 24;69(3):77-83.
https://www.doi.org/10.15585/mmwr.mm6903a5
http://www.ncbi.nlm.nih.gov/pubmed/31971933?tool=bestpractice.com
[36]Tiwari TSP, Moro PL, Acosta AM. Tetanus. In: Hall E, Wodi AP, Hamborsky J, et al, eds. Centers for Disease Control and Prevention. Epidemiology and vaccine-preventable diseases. 14th ed. Washington, D.C.: Public Health Foundation; 2021.
https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html
Clean, minor wounds do not require TIG.[3]Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018 Apr 27;67(2):1–44.
https://www.cdc.gov/mmwr/volumes/67/rr/rr6702a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/29702631?tool=bestpractice.com
[4]UK Health Security Agency. Tetanus: the green book, chapter 30. Jun 2022 [internet publication].
https://www.gov.uk/government/publications/tetanus-the-green-book-chapter-30
[36]Tiwari TSP, Moro PL, Acosta AM. Tetanus. In: Hall E, Wodi AP, Hamborsky J, et al, eds. Centers for Disease Control and Prevention. Epidemiology and vaccine-preventable diseases. 14th ed. Washington, D.C.: Public Health Foundation; 2021.
https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html
In the UK, patients with clean wounds should receive an immediate reinforcing dose of tetanus vaccine if they have not received an adequate priming course of tetanus vaccine (defined as at least 3 doses of tetanus vaccine at appropriate intervals), or their immunisation status is uncertain; otherwise, no immediate treatment is needed. For all patients, further doses should be arranged at appropriate intervals to complete the immunisation course and ensure future immunity. Patients with tetanus-prone wounds require an immediate reinforcing dose of vaccine in the following scenarios: received adequate priming course of tetanus vaccine (at least 3 doses at appropriate intervals) but last dose was more than 10 years ago, or aged 5 to 10 years old and received an adequate priming course but no preschool booster, or not received adequate priming course, or immunisation status is uncertain. Additionally, one dose of human TIG is recommended in the following: patients with tetanus-prone wounds who have not received adequate priming course or immunisation status is uncertain; patients with high-risk tetanus-prone wounds who have received adequate priming course of tetanus vaccine (at least 3 doses at appropriate intervals), but last dose was more than 10 years ago, or aged 5 to 10 years old and received an adequate priming course but no preschool booster.[4]UK Health Security Agency. Tetanus: the green book, chapter 30. Jun 2022 [internet publication].
https://www.gov.uk/government/publications/tetanus-the-green-book-chapter-30
[37]UK Health Security Agency. Tetanus: advice for health professionals. Mar 2024 [internet publication].
https://www.gov.uk/government/publications/tetanus-advice-for-health-professionals
When indicated, intramuscular TIG is the treatment of choice for prevention of tetanus and should be used if it is available.[1]Brook I. Current concepts in the management of Clostridium tetani infection. Expert Rev Anti Infect Ther. 2008 Jun;6(3):327-36.
http://www.ncbi.nlm.nih.gov/pubmed/18588497?tool=bestpractice.com
[4]UK Health Security Agency. Tetanus: the green book, chapter 30. Jun 2022 [internet publication].
https://www.gov.uk/government/publications/tetanus-the-green-book-chapter-30
[36]Tiwari TSP, Moro PL, Acosta AM. Tetanus. In: Hall E, Wodi AP, Hamborsky J, et al, eds. Centers for Disease Control and Prevention. Epidemiology and vaccine-preventable diseases. 14th ed. Washington, D.C.: Public Health Foundation; 2021.
https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html
[37]UK Health Security Agency. Tetanus: advice for health professionals. Mar 2024 [internet publication].
https://www.gov.uk/government/publications/tetanus-advice-for-health-professionals
Tetanus antitoxin (equine) is cheaper to produce and is more widely available in the developing world (it may not be available or may be difficult to access in some countries), but has a higher incidence of anaphylaxis (20% of cases) and a much shorter half-life (2 days).[19]Farrar JJ, Yen LM, Cook T, et al. Tetanus. J Neurol Neurosurg Psychiatry. 2000 Sep;69(3):292-301.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1737078/pdf/v069p00292.pdf
http://www.ncbi.nlm.nih.gov/pubmed/10945801?tool=bestpractice.com
Tetanus antitoxin (human) may also be available in some countries. If TIG cannot be sourced, guidelines in the UK recommend that the subcutaneous or intramuscular formulation of human normal immunoglobulin may be given as an alternative.[4]UK Health Security Agency. Tetanus: the green book, chapter 30. Jun 2022 [internet publication].
https://www.gov.uk/government/publications/tetanus-the-green-book-chapter-30
[37]UK Health Security Agency. Tetanus: advice for health professionals. Mar 2024 [internet publication].
https://www.gov.uk/government/publications/tetanus-advice-for-health-professionals
This strategy could also be considered in similar circumstances outside the UK.[Figure caption and citation for the preceding image starts]: Immunisation recommendations for clean and tetanus-prone woundsUK Health Security Agency. Tetanus: the green book, chapter 30. June 2022 [Citation ends].
If TIG cannot be sourced, guidelines in the UK recommend that the subcutaneous or intramuscular formulation of human normal immunoglobulin may be given intramuscularly as an alternative.
In the US, patients with clean, minor wounds who have only had up to 2 doses of a tetanus toxoid-containing vaccine or an uncertain vaccination history should be given tetanus toxoid-containing vaccine, while patients who have received ≥3 doses do not require tetanus toxoid-containing vaccine unless they have not received a dose in the last 10 years. Clean or minor wounds do not require human TIG.[3]Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018 Apr 27;67(2):1–44.
https://www.cdc.gov/mmwr/volumes/67/rr/rr6702a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/29702631?tool=bestpractice.com
[36]Tiwari TSP, Moro PL, Acosta AM. Tetanus. In: Hall E, Wodi AP, Hamborsky J, et al, eds. Centers for Disease Control and Prevention. Epidemiology and vaccine-preventable diseases. 14th ed. Washington, D.C.: Public Health Foundation; 2021.
https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html
For all other wounds, patients who have only had up to 2 doses of tetanus toxoid-containing vaccine or an uncertain vaccination history should be given tetanus toxoid-containing vaccine and TIG; patients who have received ≥3 doses do not require tetanus toxoid-containing vaccine unless they have not received a dose in the last 5 years. These patients do not require TIG.[3]Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018 Apr 27;67(2):1–44.
https://www.cdc.gov/mmwr/volumes/67/rr/rr6702a1.htm
http://www.ncbi.nlm.nih.gov/pubmed/29702631?tool=bestpractice.com
[36]Tiwari TSP, Moro PL, Acosta AM. Tetanus. In: Hall E, Wodi AP, Hamborsky J, et al, eds. Centers for Disease Control and Prevention. Epidemiology and vaccine-preventable diseases. 14th ed. Washington, D.C.: Public Health Foundation; 2021.
https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html
If a tetanus booster is indicated for wound management during pregnancy, Tdap should be administered instead of Td if the woman has not received Tdap previously.[29]Committee on Obstetric Practice, Immunization and Emerging Infections Expert Work Group. Committee Opinion No. 718: Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017 Sep;130(3):e153-7.
https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Update-on-Immunization-and-Pregnancy-Tetanus-Diphtheria-and-Pertussis-Vaccination
http://www.ncbi.nlm.nih.gov/pubmed/28832489?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: US recommendations for tetanus wound management. DTaP = diphtheria and tetanus toxoids and acellular pertussis vaccine; Tdap = tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis; Td = tetanus and diphtheria toxoids; TIG = tetanus immunoglobulin. *DTaP is recommended for children aged <7 years. Tdap is preferred to Td for persons aged ≥11 years who have not previously received Tdap. Persons aged ≥7 years who are not fully immunised against pertussis, tetanus or diphtheria should receive one dose of Tdap for wound management and as part of the catch-up series. **Immunosuppressed patients should be managed as if they were incompletely immunised (i.e., those with contaminated wounds should also receive TIG, regardless of their history of tetanus immunisation)Liang JL et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67:1-44. [Citation ends].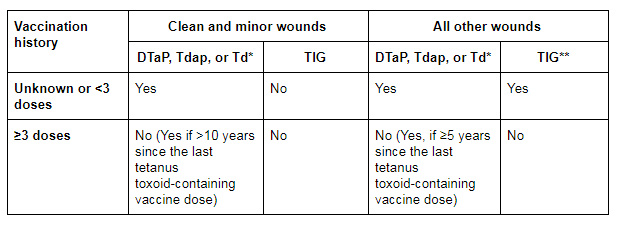
Management of clinical tetanus: supportive care
Patients should be stabilised and their airway secured to ensure adequate ventilation (which can be compromised by the muscle spasms) and to prevent aspiration of gastric contents into the lungs. Patients should be transferred to an intensive care unit. External stimulation, which can precipitate muscle spasms, should be minimised.
Airway management
There is a high risk of aspiration of gastric contents into the lungs resulting from reduced ability to cough due to muscle rigidity and sedation, pharyngeal spasms, dysphagia, gastric stasis, and increased intra-abdominal pressure during spasms. In severe tetanus, spasms may increase rapidly in frequency and duration; establishing a secure airway early is paramount before laryngeal obstruction and/or aspiration occurs.
Prolonged mechanical ventilation is often required, sometimes for weeks, and early percutaneous tracheostomy is appropriate.[47]Nakajima M, Aso S, Matsui H, et al. Clinical features and outcomes of tetanus: Analysis using a National Inpatient Database in Japan. J Crit Care. 2018 Apr;44:388-91.
http://www.ncbi.nlm.nih.gov/pubmed/29304489?tool=bestpractice.com
Patients with tetanus have increased salivation and bronchial secretions; mouth care, regular tracheal suction and chest physiotherapy are crucial to prevent secondary pulmonary infection and atelectasis. Boluses of sedation and neuromuscular blocking agents are required for these procedures to avoid stimulation.
Nutritional support
Energy demands in tetanus can be extremely high due to repeated spasms and sympathetic overdrive. Nutritional support should be initiated early, ideally by enteral feeding to maintain gastrointestinal integrity.[19]Farrar JJ, Yen LM, Cook T, et al. Tetanus. J Neurol Neurosurg Psychiatry. 2000 Sep;69(3):292-301.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1737078/pdf/v069p00292.pdf
http://www.ncbi.nlm.nih.gov/pubmed/10945801?tool=bestpractice.com
Stress ulceration
Venous thromboembolism prophylaxis
Physiotherapy
Decubitus ulcer prevention
Management of clinical tetanus: wound debridement
Wound debridement removes spores and necrotic tissue, eradicating the anaerobic conditions that facilitate clostridial growth. Antibiotic penetration into devitalised tissue is likely to be poor, emphasising the importance of adequate wound debridement.[48]Campbell JI, Lam TM, Huynh TL, et al. Microbiologic characterization and antimicrobial susceptibility of Clostridium tetani isolated from wounds of patients with clinically diagnosed tetanus. Am J Trop Med Hyg. 2009 May;80(5):827-31.
https://www.ajtmh.org/content/80/5/827.long
http://www.ncbi.nlm.nih.gov/pubmed/19407132?tool=bestpractice.com
Management of clinical tetanus: antibiotic therapy
Antibiotics halt bacterial replication and thereby reduce the production of new toxins. Metronidazole has superseded benzylpenicillin as the antimicrobial of choice for the treatment of tetanus. Benzylpenicillin has traditionally been used for this purpose.[19]Farrar JJ, Yen LM, Cook T, et al. Tetanus. J Neurol Neurosurg Psychiatry. 2000 Sep;69(3):292-301.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1737078/pdf/v069p00292.pdf
http://www.ncbi.nlm.nih.gov/pubmed/10945801?tool=bestpractice.com
However, benzylpenicillin is structurally similar to gamma-aminobutyric acid (GABA) and competitively antagonises this neurotransmitter, an action that could potentiate the effects of tetanus toxin in inhibiting release of GABA into the synaptic cleft and enhancing central nervous system excitability.[19]Farrar JJ, Yen LM, Cook T, et al. Tetanus. J Neurol Neurosurg Psychiatry. 2000 Sep;69(3):292-301.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1737078/pdf/v069p00292.pdf
http://www.ncbi.nlm.nih.gov/pubmed/10945801?tool=bestpractice.com
Evidence suggests that, compared with benzylpenicillin, metronidazole was associated with reduced mortality.[49]Ahmadsyah I, Salim A. Treatment of tetanus: an open study to compare the efficacy of procaine penicillin and metronidazole. Br Med J (Clin Res Ed). 1985 Sep 7;291(6496):64.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1417474/pdf/bmjcred00464-0036.pdf
http://www.ncbi.nlm.nih.gov/pubmed/3928066?tool=bestpractice.com
Other evidence indicates no difference in mortality, but that metronidazole is associated with a lower requirement for muscle relaxants and sedatives.[50]Yen LM, Dao LM, Day NPJ, et al. Management of tetanus: a comparison of penicillin and metronidazole. Paper presented at: Symposium of antimicrobial resistance in southern Viet Nam; 1997; Ho Chi Minh City, Vietnam. This difference may be attributable to the GABA antagonist effect of benzylpenicillin. Alternative antibiotics include clindamycin, tetracycline, and vancomycin.[1]Brook I. Current concepts in the management of Clostridium tetani infection. Expert Rev Anti Infect Ther. 2008 Jun;6(3):327-36.
http://www.ncbi.nlm.nih.gov/pubmed/18588497?tool=bestpractice.com
Management of clinical tetanus: use of human TIG
US guidelines recommend that intramuscular TIG is administered to patients with clinical tetanus.[1]Brook I. Current concepts in the management of Clostridium tetani infection. Expert Rev Anti Infect Ther. 2008 Jun;6(3):327-36.
http://www.ncbi.nlm.nih.gov/pubmed/18588497?tool=bestpractice.com
[36]Tiwari TSP, Moro PL, Acosta AM. Tetanus. In: Hall E, Wodi AP, Hamborsky J, et al, eds. Centers for Disease Control and Prevention. Epidemiology and vaccine-preventable diseases. 14th ed. Washington, D.C.: Public Health Foundation; 2021.
https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html
It should be administered as soon as possible after the injury.[1]Brook I. Current concepts in the management of Clostridium tetani infection. Expert Rev Anti Infect Ther. 2008 Jun;6(3):327-36.
http://www.ncbi.nlm.nih.gov/pubmed/18588497?tool=bestpractice.com
Passive immunisation neutralises unbound toxin, reducing the duration and severity of tetanus.
TIG (half-life 24.5 to 31.5 days) should be used if it is available.[1]Brook I. Current concepts in the management of Clostridium tetani infection. Expert Rev Anti Infect Ther. 2008 Jun;6(3):327-36.
http://www.ncbi.nlm.nih.gov/pubmed/18588497?tool=bestpractice.com
[36]Tiwari TSP, Moro PL, Acosta AM. Tetanus. In: Hall E, Wodi AP, Hamborsky J, et al, eds. Centers for Disease Control and Prevention. Epidemiology and vaccine-preventable diseases. 14th ed. Washington, D.C.: Public Health Foundation; 2021.
https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html
However, tetanus antitoxin (equine) is more widely available in the developing world (it may not be available or may be difficult to access in some countries), but has a higher incidence of anaphylaxis (20% of cases) and a much shorter half-life (2 days).[19]Farrar JJ, Yen LM, Cook T, et al. Tetanus. J Neurol Neurosurg Psychiatry. 2000 Sep;69(3):292-301.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1737078/pdf/v069p00292.pdf
http://www.ncbi.nlm.nih.gov/pubmed/10945801?tool=bestpractice.com
Tetanus antitoxin (human) may also be available in some countries.
Guidelines in the UK recommend the use of intravenous human normal immunoglobulin rather than intramuscular TIG, for the treatment of clinical tetanus.[4]UK Health Security Agency. Tetanus: the green book, chapter 30. Jun 2022 [internet publication].
https://www.gov.uk/government/publications/tetanus-the-green-book-chapter-30
[37]UK Health Security Agency. Tetanus: advice for health professionals. Mar 2024 [internet publication].
https://www.gov.uk/government/publications/tetanus-advice-for-health-professionals
This strategy could also be considered in similar circumstances outside the UK.
An intravenous preparation of TIG is no longer available in the UK or the US.
Management of clinical tetanus: active immunisation with tetanus vaccine
Patients with clinical tetanus should receive immunisation with tetanus toxoid-containing vaccine to stimulate long-term humoral and cellular immunity. In addition, it is thought that tetanus toxoid saturates ganglioside receptors, blocking the binding of wild-type toxin.[19]Farrar JJ, Yen LM, Cook T, et al. Tetanus. J Neurol Neurosurg Psychiatry. 2000 Sep;69(3):292-301.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1737078/pdf/v069p00292.pdf
http://www.ncbi.nlm.nih.gov/pubmed/10945801?tool=bestpractice.com
Toxoid should be injected at a different site from immunoglobulin so that it is not 'neutralised' by the passive immunisation.
Management of clinical tetanus: control of muscle spasms
Muscle spasms are extremely painful and potentially life-threatening if they cause airway compromise or respiratory failure. Benzodiazepines have been the mainstay of controlling muscle spasms, and in addition have anticonvulsant, sedative, and anxiolytic effects. They block an endogenous inhibitor at the GABAA receptor. Diazepam is often used.[1]Brook I. Current concepts in the management of Clostridium tetani infection. Expert Rev Anti Infect Ther. 2008 Jun;6(3):327-36.
http://www.ncbi.nlm.nih.gov/pubmed/18588497?tool=bestpractice.com
High doses may necessitate ventilatory assistance and have been associated with lactic acidosis due to the excipient propylene glycol.[51]Kapoor W, Carey P, Karpf M. Induction of lactic acidosis with intravenous diazepam in a patient with tetanus. Arch Intern Med. 1981 Jun;141(7):944-5.
http://www.ncbi.nlm.nih.gov/pubmed/7235819?tool=bestpractice.com
Diazepam metabolites are active with long half-lives (desmethyldiazepam has a half-life of >100 hours), and for this reason midazolam infusions may be preferred in adults.[52]Attygalle D, Rodrigo N. New trends in the management of tetanus. Expert Rev Anti Infect Ther. 2004 Feb;2(1):73-84.
http://www.ncbi.nlm.nih.gov/pubmed/15482173?tool=bestpractice.com
[53]Gyasi HK, Fahr J, Kurian E, et al. Midazolam for prolonged intravenous sedation in patients with tetanus. Middle East J Anesthesiol. 1993 Jun;12(2):135-41.
http://www.ncbi.nlm.nih.gov/pubmed/8413057?tool=bestpractice.com
In children, diazepam may cause significant respiratory depression; therefore, midazolam or lorazepam may be preferred.
There is some evidence that diazepam is more effective in treating tetanus than alternative sedatives such as phenothiazines and barbiturates.[54]Okoromah CN, Lesi FE. Diazepam for treating tetanus. Cochrane Database Syst Rev. 2004 Jan 26;(1):CD003954.
http://www.ncbi.nlm.nih.gov/pubmed/14974046?tool=bestpractice.com
However, the studies concerned were limited by their small size, lack of data on drug safety, and other methodological drawbacks. Large multi-centre randomised controlled trials are needed to establish whether diazepam is superior to phenobarbital and chlorpromazine.
Some patients require paralysis with non-depolarising neuromuscular blocking agents in addition to sedation. Traditionally pancuronium was used, although this was known to potentially aggravate autonomic instability.[55]Buchanan N, Cane RD, Wolfson G, et al. Autonomic dysfunction in tetanus: the effects of a variety of therapeutic agents, with special reference to morphine. Intensive Care Med. 1979 May;5(2):65-8.
http://www.ncbi.nlm.nih.gov/pubmed/156745?tool=bestpractice.com
Vecuronium and rocuronium are associated with less autonomic disturbance, and are preferred.
Baclofen stimulates postsynaptic GABAB receptors and has been found to improve muscle spasms when given by intrathecal bolus or infusion, but only in a few small studies.[56]Engrand N, Vilain G, Rouamba A, et al. Value of intrathecal baclofen in the treatment of severe tetanus in the tropical milieu. Med Trop (Mars). 2000;60(4):385-8. [In French]
http://www.ncbi.nlm.nih.gov/pubmed/11436597?tool=bestpractice.com
[57]Saissy JM, Demaziere J, Vitris M, et al. Treatment of severe tetanus by intrathecal injections of baclofen without artificial ventilation. Intensive Care Med. 1992;18(4):241-4.
http://www.ncbi.nlm.nih.gov/pubmed/1430590?tool=bestpractice.com
[58]Boots RJ, Lipman J, O'Callaghan J, et al. The treatment of tetanus with intrathecal baclofen. Anaesth Intensive Care. 2000 Aug;28(4):438-42.
http://www.ncbi.nlm.nih.gov/pubmed/10969374?tool=bestpractice.com
[59]Dressnandt J, Konstanzer A, Weinzierl FX, et al. Intrathecal baclofen in tetanus: four cases and a review of reported cases. Intensive Care Med. 1997 Aug;23(8):896-902.
http://www.ncbi.nlm.nih.gov/pubmed/9310810?tool=bestpractice.com
In a retrospective outcome study from a single centre in Portugal during 1998 to 2003, intrathecal baclofen was given as an initial bolus, followed by a continuous infusion.[60]Santos ML, Mota-Miranda A, Alves-Pereira A, et al. Intrathecal baclofen for the treatment of tetanus. Clin Infect Dis. 2004 Feb 1;38(3):321-8.
https://cid.oxfordjournals.org/content/38/3/321.full
http://www.ncbi.nlm.nih.gov/pubmed/14727200?tool=bestpractice.com
This controlled spasms and rigidity in 21 of 22 patients with grade 3 tetanus. Most patients required therapy for at least 3 weeks (range 8 to 30 days). One patient developed meningitis secondary to infection of the intrathecal catheter. Intrathecal baclofen has a narrow therapeutic range and considerable inter-individual pharmacodynamic variability.[56]Engrand N, Vilain G, Rouamba A, et al. Value of intrathecal baclofen in the treatment of severe tetanus in the tropical milieu. Med Trop (Mars). 2000;60(4):385-8. [In French]
http://www.ncbi.nlm.nih.gov/pubmed/11436597?tool=bestpractice.com
Treatment with intrathecal baclofen should only be considered under specialist guidance and administration.
Management of clinical tetanus: autonomic dysfunction
Autonomic dysfunction is extremely difficult to control. It arises in patients with severe disease, usually in the second week of illness.
Magnesium sulfate is a presynaptic neuromuscular blocker, blocks catecholamine release from nerves and the adrenal medulla, and reduces receptor responsiveness to catecholamines. It is also an anticonvulsant and a calcium antagonist in the myocardium.[19]Farrar JJ, Yen LM, Cook T, et al. Tetanus. J Neurol Neurosurg Psychiatry. 2000 Sep;69(3):292-301.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1737078/pdf/v069p00292.pdf
http://www.ncbi.nlm.nih.gov/pubmed/10945801?tool=bestpractice.com
Electromyogram studies have suggested that magnesium tends to spare the respiratory muscles, although at high doses ventilation may be depressed, mandating ventilatory support.[52]Attygalle D, Rodrigo N. New trends in the management of tetanus. Expert Rev Anti Infect Ther. 2004 Feb;2(1):73-84.
http://www.ncbi.nlm.nih.gov/pubmed/15482173?tool=bestpractice.com
[61]Lee C, Zhang X, Kwan WF. Electromyographic and mechanomyographic characteristics of neuromuscular block by magnesium sulphate in the pig. Br J Anaesth. 1996 Feb;76(2):278-83.
http://bja.oxfordjournals.org/content/76/2/278.full.pdf+html
http://www.ncbi.nlm.nih.gov/pubmed/8777111?tool=bestpractice.com
Magnesium sulfate has previously been reported to be both an effective adjunct in controlling autonomic disturbance in heavily sedated patients with severe tetanus and successful in relieving spasms in non-ventilated patients.[62]James MFM, Manson EDM. The use of magnesium sulphate infusions in the management of very severe tetanus. Intensive Care Med. 1985;11(1):5-12.
http://www.ncbi.nlm.nih.gov/pubmed/3968303?tool=bestpractice.com
[63]Lipman J, James MFM, Erskine J, et al. Autonomic dysfunction in severe tetanus: magnesium sulfate as an adjunct to deep sedation. Crit Care Med. 1987 Oct;15(10):987-8.
http://www.ncbi.nlm.nih.gov/pubmed/3652717?tool=bestpractice.com
[64]Attygalle D, Rodrigo N. Magnesium sulphate for control of spasms in severe tetanus. Can we avoid sedation and artificial ventilation? Anaesthesia. 1997 Oct;52(10):956-62.
http://www.ncbi.nlm.nih.gov/pubmed/9370837?tool=bestpractice.com
One randomised controlled trial found that magnesium sulfate significantly reduced the requirement for other drugs (e.g., midazolam) used for the control of muscle spasms and showed that patients are less likely to need verapamil for cardiovascular instability, when compared with placebo.[65]Thwaites CL, Yen LM, Loan HT, et al. Magnesium sulphate for treatment of severe tetanus: a randomised controlled trial. Lancet. 2006 Oct 21;368(9545):1436-43.
http://www.ncbi.nlm.nih.gov/pubmed/17055945?tool=bestpractice.com
There was no difference in the need for mechanical ventilation.[65]Thwaites CL, Yen LM, Loan HT, et al. Magnesium sulphate for treatment of severe tetanus: a randomised controlled trial. Lancet. 2006 Oct 21;368(9545):1436-43.
http://www.ncbi.nlm.nih.gov/pubmed/17055945?tool=bestpractice.com
An earlier, small prospective observational study suggested that magnesium sulfate reduces not only the use of neuromuscular blocking agents to control severe spasms but also the requirement for mechanical ventilation when compared with historical controls.[66]Attygalle D, Rodrigo N. Magnesium as first line therapy in the management of tetanus: a prospective study of 40 patients. Anaesthesia. 2002 Aug;57(8):811-7.
http://www.ncbi.nlm.nih.gov/pubmed/12133096?tool=bestpractice.com
Conflicting results may reflect differences in study design and magnesium administration.[65]Thwaites CL, Yen LM, Loan HT, et al. Magnesium sulphate for treatment of severe tetanus: a randomised controlled trial. Lancet. 2006 Oct 21;368(9545):1436-43.
http://www.ncbi.nlm.nih.gov/pubmed/17055945?tool=bestpractice.com
A loading dose of 5 g of magnesium sulfate given intravenously over 20 minutes has been advocated, followed by magnesium infusion, the rate of which is titrated to the control of spasms and rigidity.[52]Attygalle D, Rodrigo N. New trends in the management of tetanus. Expert Rev Anti Infect Ther. 2004 Feb;2(1):73-84.
http://www.ncbi.nlm.nih.gov/pubmed/15482173?tool=bestpractice.com
The aim is not to completely abolish muscle rigidity, but to reduce it to a level that is acceptable to the patient and allows swallowing of saliva, mouth care, and limb physiotherapy. Doses as high as 4 to 5 g/hour may be necessary, with monitoring for respiratory depression. One meta-analysis of 3 controlled trials found no reduction in mortality for patients treated with magnesium sulfate compared with placebo or diazepam therapy. Conclusions about the effects of magnesium on duration of intensive care stay, duration of hospital stay, and requirement for ventilatory support could not be drawn due to large methodological differences between the studies included.[67]Rodrigo C, Samarakoon L, Fernando SD, et al. A meta-analysis of magnesium for tetanus. Anaesthesia. 2012 Dec;67(12):1370-4.
http://www.ncbi.nlm.nih.gov/pubmed/23033859?tool=bestpractice.com
Sedation helps to reduce autonomic instability, and both benzodiazepines and morphine sulfate are useful in this regard. Morphine sulfate reduces sympathetic tone in the heart and the vascular system, improving cardiovascular stability without compromising cardiac performance.[1]Brook I. Current concepts in the management of Clostridium tetani infection. Expert Rev Anti Infect Ther. 2008 Jun;6(3):327-36.
http://www.ncbi.nlm.nih.gov/pubmed/18588497?tool=bestpractice.com
[68]Rocke DA, Wesley AG, Pather M, et al. Morphine in tetanus--the management of sympathetic nervous system overactivity. S Afr Med J. 1986 Nov 22;70(11):666-8.
http://www.ncbi.nlm.nih.gov/pubmed/3787380?tool=bestpractice.com
Beta-blockade may be required in further management of the autonomic instability. Choice and dosing of a beta-blocker should be decided in consultation with a specialist. Pure beta-blockade, with propranolol, has been associated with sudden death.[69]Buchanan N, Smit L, Cane RD, et al. Sympathetic overactivity in tetanus: fatality associated with propranolol. Br Med J. 1978 Jul 22;2(6132):254-5.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1606368/pdf/brmedj00136-0030b.pdf
http://www.ncbi.nlm.nih.gov/pubmed/678897?tool=bestpractice.com
Atropine, clonidine, and epidural/spinal bupivacaine have been reported to improve autonomic disturbance in either individual patients or very small series. Larger trials are needed to adequately assess outcome measures for these treatments.[70]Dolar D. The use of continuous atropine infusion in the management of tetanus. Intensive Care Med. 1992;18(1):26-31.
http://www.ncbi.nlm.nih.gov/pubmed/1578043?tool=bestpractice.com
[71]Sutton DN, Tremlett MR, Woodcock TE, et al. Management of autonomic dysfunction in severe tetanus: the use of magnesium sulphate and clonidine. Intensive Care Med. 1990;16(2):75-80.
http://www.ncbi.nlm.nih.gov/pubmed/2094230?tool=bestpractice.com
[72]Gregorakos L, Kerezoudi E, Dimopoulos G, et al. Management of blood pressure instability in severe tetanus: the use of clonidine. Intensive Care Med. 1997 Aug;23(8):893-5.
http://www.ncbi.nlm.nih.gov/pubmed/9310809?tool=bestpractice.com
[73]Southorn PA, Blaise GA. Treatment of tetanus-induced autonomic nervous system dysfunction with continuous epidural blockade. Crit Care Med. 1986 Mar;14(3):251-2.
http://www.ncbi.nlm.nih.gov/pubmed/3943342?tool=bestpractice.com
[74]Shibuya M, Sugimoto H, Sugimoto T, et al. The use of continuous spinal anesthesia in severe tetanus with autonomic disturbance. J Trauma. 1989 Oct;29(10):1423-9.
http://www.ncbi.nlm.nih.gov/pubmed/2810420?tool=bestpractice.com
[75]Freshwater-Turner D, Udy A, Lipman J, et al. Autonomic dysfunction in tetanus - what lessons can be learnt with special reference to alpha-2 agonists? Anaesthesia. 2007 Oct;62(10):1066-70.
http://www.ncbi.nlm.nih.gov/pubmed/17845661?tool=bestpractice.com

