Treatment usually consists of the following components:
Brief intervention, particularly in unhealthy alcohol use and mild alcohol use disorder
Individual therapy
Psychosocial support
Drug treatment to support reduced alcohol intake and abstinence
Ongoing support to help the patient maintain their goals regarding alcohol intake
Patients may benefit from a combination of interventions, and the use of one should not preclude the use of another.[77]Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA. 2018 Aug 28;320(8):815-24.
http://www.ncbi.nlm.nih.gov/pubmed/30167705?tool=bestpractice.com
[78]Ray LA, Meredith LR, Kiluk BD, et al. Combined pharmacotherapy and cognitive behavioral therapy for adults with alcohol or substance use disorders: a systematic review and meta-analysis. JAMA Netw Open. 2020 Jun 1;3(6):e208279.
https://www.doi.org/10.1001/jamanetworkopen.2020.8279
http://www.ncbi.nlm.nih.gov/pubmed/32558914?tool=bestpractice.com
Goals of treatment may include abstinence, reduction or moderation of alcohol use, or other elements of harm reduction, and should be agreed between patient and clinician. The patient’s legal obligations, and risk of harm to self or others from alcohol use, should also be discussed.[51]Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018 Jan 1;175(1):86-90.
https://psychiatryonline.org/doi/book/10.1176/appi.books.9781615371969
http://www.ncbi.nlm.nih.gov/pubmed/29301420?tool=bestpractice.com
The appropriate level of care for a patient is determined via six dimensions:
Withdrawal risk
Biomedical conditions or complications
Stability of co-occurring mood disorders and cognitive status
Readiness to change
Likelihood of return to use
Living environment and psychosocial support.
Patients who are medically unstable or do not meet their goals with outpatient management can be stepped up to a higher level of care.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
Lack of access to a higher level of care, however, should not prevent clinicians from offering drug treatment, support services, and harm reduction strategies.
Engagement and retention in any substance use treatment can be a major clinical challenge; it is recommended that healthcare providers proactively engage people who would benefit from treatment at all stages of readiness for change, including those who are uninterested or ambivalent about receiving treatment.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
The following strategies are amongst those recommended by the American Society of Addiction Medicine to improve treatment engagement and retention:[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
Cultivate patient trust by creating a welcoming, non-judgemental and trauma-sensitive environment
Do not require abstinence as a condition of treatment initiation or retention
Build connections to people with a substance use disorder who are not currently seeking treatment
Seek to re-engage individuals who disengage from care
Develop treatment plans that are responsive to the individual’s needs and priorities
Address the physical, psychological, social, and cultural factors that contribute to substance use and recovery
Alcohol use treatment programmes should provide integrated care and address co-occurring mental and physical health conditions, as required, which may influence effective participation in treatment.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
[Figure caption and citation for the preceding image starts]: Treatment overviewCreated by BMJ Knowledge Centre [Citation ends].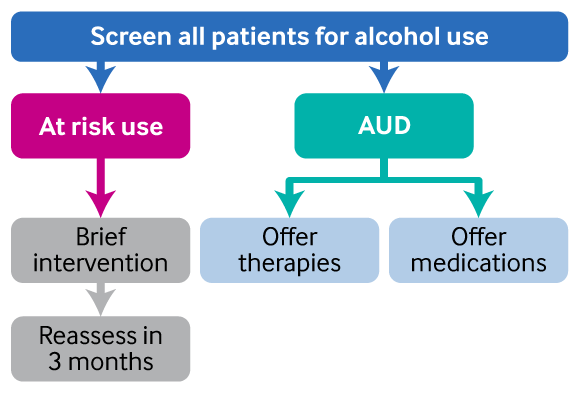
Management of withdrawal
One of the most life-threatening complications of alcohol use disorder is alcohol withdrawal. As a result, telling patients who use alcohol daily to stop drinking abruptly could be dangerous. At the time of assessment, patients should be assessed with a structured instrument, such as the revised Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar), to determine the severity of the alcohol withdrawal syndrome (AWS).[79]Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar). Br J Addiction. 1989;84:1353-1357.
http://www.ncbi.nlm.nih.gov/pubmed/2597811?tool=bestpractice.com
Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA- Ar)
Opens in new window
Severity guides the management of alcohol withdrawal, which centres on benzodiazepines as first-line drug treatment, close monitoring, correction of fluid and electrolyte imbalance, and consideration of thiamine (vitamin B1) to prevent Wernicke's encephalopathy and Korsakoff syndrome. See Alcohol withdrawal.
Brief interventions
People with unhealthy alcohol use that do not meet alcohol use disorder criteria can often be treated with a brief intervention. These interventions are typically 5-15 minutes long, follow a structured outline, provide education, and may or may not include follow-up treatment. In patients with at-risk drinking, these interventions reduce total alcohol consumed.[42]US Department of Veterans Affairs. VA/DOD clinical practice guidelines: management of substance use disorders. 2021 [internet publication].
https://www.healthquality.va.gov/guidelines/MH/sud
[80]Bertholet N, Daeppen JB, Wietlisbach V, et al. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med. 2005 May 9;165(9):986-95.
https://www.doi.org/10.1001/archinte.165.9.986
http://www.ncbi.nlm.nih.gov/pubmed/15883236?tool=bestpractice.com
[81]Álvarez-Bueno C, Rodríguez-Martín B, García-Ortiz L, et al. Effectiveness of brief interventions in primary health care settings to decrease alcohol consumption by adult non-dependent drinkers: a systematic review of systematic reviews. Prev Med. 2015 Jul;76(suppl):S33-8.
http://www.ncbi.nlm.nih.gov/pubmed/25514547?tool=bestpractice.com
Most studies excluded patients with moderate to severe alcohol use disorder, and evidence for the effectiveness of brief intervention without referral to treatment in this population, as well as in those with multiple substance use disorders, is lacking.
Brief interventions can consist of one or more sessions in the clinic or hospital setting (e.g., emergency department or inpatient unit), during which education and feedback about the patient's alcohol use and its consequences are provided in a supportive and empathic fashion.
[  ]
What are the effects of brief interventions in heavy alcohol users admitted to general hospital wards?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.593/fullShow me the answer Brief interventions may be improved by follow-up; for instance, one multi-site randomised controlled trial (RCT) in the trauma setting showed improvement in several drinking measures at up to 12 months for patients who received an individualised follow-up phone call in addition to brief motivational interviewing, compared with those who just received the interview.[82]Field C, Walters S, Marti CN, et al. A multisite randomized controlled trial of brief intervention to reduce drinking in the trauma care setting: how brief is brief? Ann Surg. 2014 May;259(5):873-80.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3984362
http://www.ncbi.nlm.nih.gov/pubmed/24263324?tool=bestpractice.com
A formal means of assessing the effectiveness of the plan and follow-up visits with the clinician should be part of the plan.[83]Kaner EF, Beyer FR, Muirhead C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2018 Feb 24;(2):CD004148.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD004148.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/29476653?tool=bestpractice.com
Though brief interventions are easy to administer, they go beyond simply telling patients to reduce their alcohol use: in studies of their effectiveness, clinicians were trained to provide structured feedback based on an individual’s risk and desire to change their behaviour. One commonly used structure is the FRAMES method: Feedback, Responsibility, Advice, Menu of options, Empathic style, Self-efficacy.
]
What are the effects of brief interventions in heavy alcohol users admitted to general hospital wards?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.593/fullShow me the answer Brief interventions may be improved by follow-up; for instance, one multi-site randomised controlled trial (RCT) in the trauma setting showed improvement in several drinking measures at up to 12 months for patients who received an individualised follow-up phone call in addition to brief motivational interviewing, compared with those who just received the interview.[82]Field C, Walters S, Marti CN, et al. A multisite randomized controlled trial of brief intervention to reduce drinking in the trauma care setting: how brief is brief? Ann Surg. 2014 May;259(5):873-80.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3984362
http://www.ncbi.nlm.nih.gov/pubmed/24263324?tool=bestpractice.com
A formal means of assessing the effectiveness of the plan and follow-up visits with the clinician should be part of the plan.[83]Kaner EF, Beyer FR, Muirhead C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2018 Feb 24;(2):CD004148.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD004148.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/29476653?tool=bestpractice.com
Though brief interventions are easy to administer, they go beyond simply telling patients to reduce their alcohol use: in studies of their effectiveness, clinicians were trained to provide structured feedback based on an individual’s risk and desire to change their behaviour. One commonly used structure is the FRAMES method: Feedback, Responsibility, Advice, Menu of options, Empathic style, Self-efficacy.
[Figure caption and citation for the preceding image starts]: FRAMES method for brief interventionAdapted by Best Practice topic authors from: Bien TH et al. Addiction. 1993 Mar;88(3):315-35 [Citation ends].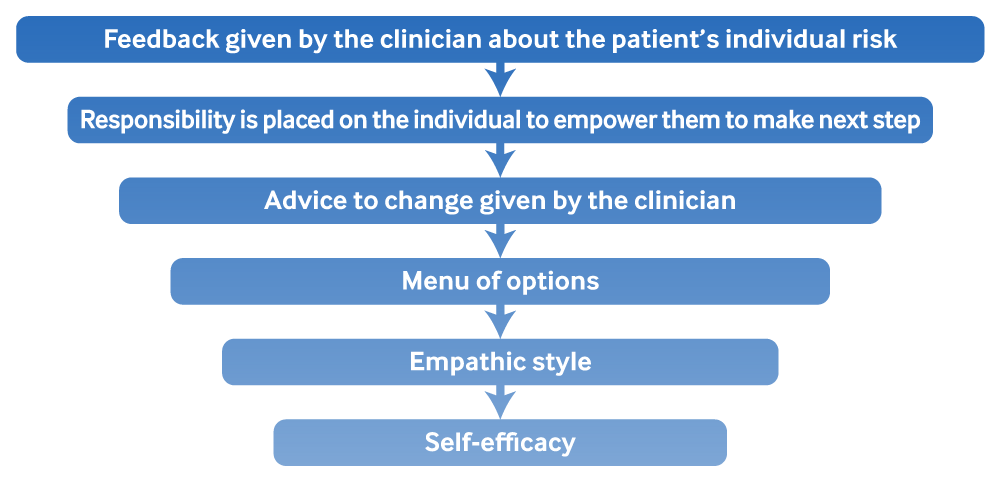
Motivational interviewing
Motivational interviewing (MI, a technique that assists the patient in identifying their own goals) can be an effective, patient-centred way of engaging patients in treatment and reducing substance use.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
[42]US Department of Veterans Affairs. VA/DOD clinical practice guidelines: management of substance use disorders. 2021 [internet publication].
https://www.healthquality.va.gov/guidelines/MH/sud
[84]Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behavior. New York, NY: Guilford Press; 1991. MI requires clinicians to avoid confronting, labelling, or directing the patient, but rather to elicit the patient’s ambivalence and support his/her motivation to change through open-ended questions, affirmations, reflective statements, and summarising. It can be implemented effectively in community settings if clinicians are trained in this approach.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
[42]US Department of Veterans Affairs. VA/DOD clinical practice guidelines: management of substance use disorders. 2021 [internet publication].
https://www.healthquality.va.gov/guidelines/MH/sud
There are quality limitations, however, to the evidence on MI. One Cochrane systematic review found that MI may be effective in reducing substance use in the immediate- to short-term (compared with no treatment), but had no effect on readiness to change behaviour.[85]Schwenker R, Dietrich CE, Hirpa S, et al. Motivational interviewing for substance use reduction. Cochrane Database Syst Rev. 2023 Dec 12;12(12):CD008063.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10714668
http://www.ncbi.nlm.nih.gov/pubmed/38084817?tool=bestpractice.com
Studies were heterogeneous, with no evidence specific to alcohol use.
Psychosocial interventions
Non-pharmacological approaches can be useful for many patients with alcohol use disorder, as they provide strategies to help patients avoid returning to alcohol use, enhance self-efficacy, and reduce the impact of stressors that can precipitate return to use. Interventions may be individual or group-based. There is no strong evidence for any one approach, and therefore patient preference and local availability should guide treatment choice.[42]US Department of Veterans Affairs. VA/DOD clinical practice guidelines: management of substance use disorders. 2021 [internet publication].
https://www.healthquality.va.gov/guidelines/MH/sud
[86]Wendt DC, Gone JP. Group therapy for substance use disorders: a survey of clinician practices. J Groups Addict Recover. 2017;12(4):243-59.
http://www.ncbi.nlm.nih.gov/pubmed/30546274?tool=bestpractice.com
[87]World Health Organization. Mental Health Gap Action Programme (mhGAP) guideline for mental, neurological and substance use disorders, third edition. Nov 2023 [internet publication].
https://www.who.int/publications/i/item/9789240084278
US guidelines from the Department of Veterans Affairs/Department of Defense (VA/DoD) suggest the following as first-line options that have demonstrated modest effect on alcohol consumption and recovery:[42]US Department of Veterans Affairs. VA/DOD clinical practice guidelines: management of substance use disorders. 2021 [internet publication].
https://www.healthquality.va.gov/guidelines/MH/sud
Cognitive behavioural therapy (CBT, assists patients in coping with thoughts of return to alcohol use and negative cognitions)
Behavioural couples therapy (BCT, aims to improve relationship functioning with goals to reduce alcohol use with partner support)
Community reinforcement approach (CRA, comprehensive cognitive behavioural approach that addresses environmental contingencies that influence drinking behaviour)
Motivational enhancement therapy (MET, based on the principles of motivational interviewing but using a structured, individualised plan incorporating assessment and feedback to enhance self-efficacy)
12-step facilitation (TSF, systematised approach that aims to enhance engagement in Alcoholics Anonymous [AA] or other 12-step based self-help groups)
Outpatient and intensive outpatient treatment programmes typically include treatment sessions scheduled from once- or twice-weekly to several times a week, with treatment extending over several weeks to months (or longer).
AA, founded in 1939, is the most common programme. Its primary goal is to help individuals maintain total abstinence from alcohol and other addictive substances.
Alcoholics Anonymous
Opens in new window
[  ]
Does RCT evidence indicate that manualized Alcoholics Anonymous (AA) or other 12‐step programs offer benefit over alternative interventions for people with alcohol use disorder?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2976/fullShow me the answer[Evidence A]c68ec014-6900-4a8c-a372-df717225e5bfccaADoes RCT evidence indicate that manualised Alcoholics Anonymous (AA) or other 12‐step programmes offer benefit over alternative interventions for people with alcohol-use disorder? Self-help programmes such as AA can provide valuable additional support for many patients and their families. Patients indicating benefit from support group attendance should be encouraged to attend the group meetings over an extended timeframe; some patients may benefit from attending indefinitely. In one large RCT, AA improved drinking outcomes, largely through improvements in adaptive social network changes and social self-efficacy.[88]Kelly JF, Hoeppner B, Stout RL, et al. Determining the relative importance of the mechanisms of behavior change within Alcoholics Anonymous: a multiple mediator analysis. Addiction. 2012 Feb;107(2):289-99.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3242865
http://www.ncbi.nlm.nih.gov/pubmed/21917054?tool=bestpractice.com
Each AA group is independent, so patients should be encouraged to try several if one is not a good fit. For those who have negative experiences or views of AA, it is important that clinicians are aware of alternatives, such as Smart Recovery groups.
]
Does RCT evidence indicate that manualized Alcoholics Anonymous (AA) or other 12‐step programs offer benefit over alternative interventions for people with alcohol use disorder?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2976/fullShow me the answer[Evidence A]c68ec014-6900-4a8c-a372-df717225e5bfccaADoes RCT evidence indicate that manualised Alcoholics Anonymous (AA) or other 12‐step programmes offer benefit over alternative interventions for people with alcohol-use disorder? Self-help programmes such as AA can provide valuable additional support for many patients and their families. Patients indicating benefit from support group attendance should be encouraged to attend the group meetings over an extended timeframe; some patients may benefit from attending indefinitely. In one large RCT, AA improved drinking outcomes, largely through improvements in adaptive social network changes and social self-efficacy.[88]Kelly JF, Hoeppner B, Stout RL, et al. Determining the relative importance of the mechanisms of behavior change within Alcoholics Anonymous: a multiple mediator analysis. Addiction. 2012 Feb;107(2):289-99.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3242865
http://www.ncbi.nlm.nih.gov/pubmed/21917054?tool=bestpractice.com
Each AA group is independent, so patients should be encouraged to try several if one is not a good fit. For those who have negative experiences or views of AA, it is important that clinicians are aware of alternatives, such as Smart Recovery groups.
When provided alongside pharmacological treatment or other first-line psychosocial interventions, the VA/DoD reports evidence to support options of CBT, MET or TSF.[42]US Department of Veterans Affairs. VA/DOD clinical practice guidelines: management of substance use disorders. 2021 [internet publication].
https://www.healthquality.va.gov/guidelines/MH/sud
MET strategies, in particular, have been recognised to encourage engagement and retention in care.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
One Cochrane systematic review found moderate-certainty evidence that, compared with psychosocial therapy alone, adding pharmacological treatment was safe and reduced the number of ‘heavy drinkers’, though an effect on other outcomes (such as volume and frequency of consumption or treatment retention) was unclear.[89]Minozzi S, La Rosa GRM, Salis F, et al. Combined pharmacological and psychosocial interventions for alcohol use disorder. Cochrane Database Syst Rev. 2025 Mar 20;3(3):CD015673.
http://www.ncbi.nlm.nih.gov/pubmed/40110869?tool=bestpractice.com
It was also uncertain whether adjunctive psychosocial therapy gave benefit compared with pharmacological treatment alone. Most studies have evaluated CBT adjunctive to naltrexone, in people without comorbid mental health or substance use disorder.[89]Minozzi S, La Rosa GRM, Salis F, et al. Combined pharmacological and psychosocial interventions for alcohol use disorder. Cochrane Database Syst Rev. 2025 Mar 20;3(3):CD015673.
http://www.ncbi.nlm.nih.gov/pubmed/40110869?tool=bestpractice.com
An earlier meta-analysis of six studies had found the addition of manualised CBT did not improve return-to-use rates when added to naltrexone therapy.[90]Agosti V, Nunes EV, O'Shea D. Do manualized psychosocial interventions help reduce relapse among alcohol-dependent adults treated with naltrexone or placebo? A meta-analysis. Am J Addict. 2012 Nov-Dec;21(6):501-7.
http://www.ncbi.nlm.nih.gov/pubmed/23082827?tool=bestpractice.com
The following table summarises psychological interventions in the outpatient setting.
[Figure caption and citation for the preceding image starts]: Non-pharmacological approaches can be useful for many patients with alcohol use disorder, as they provide strategies to help patients avoid returning to alcohol use, enhance self-efficacy, and reduce the impact of stressors that can precipitate return to useTable created by authors of the Best Practice topic [Citation ends].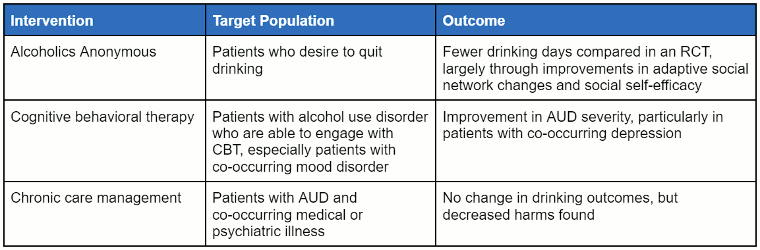
Pharmacological treatment
Pharmacological treatment can help support abstinence, reduce alcohol consumed, and reduce the number of heavy drinking days, all of which are likely to reduce harm from alcohol use.[77]Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA. 2018 Aug 28;320(8):815-24.
http://www.ncbi.nlm.nih.gov/pubmed/30167705?tool=bestpractice.com
Nonetheless, drug therapy for alcohol use disorder remains underutilised because of lack of clinician and patient familiarity, and perhaps a tendency to discount outcomes other than abstinence as worthwhile clinical goals. Three drugs - naltrexone, acamprosate, and disulfiram - are approved by the US Food and Drug Administration (FDA) for the treatment of alcohol use disorder. Naltrexone, acamprosate, and disulfiram have been studied in patients with moderate to severe alcohol use disorder, but may be offered to patients with mild alcohol use disorder on a case-by-case basis.[42]US Department of Veterans Affairs. VA/DOD clinical practice guidelines: management of substance use disorders. 2021 [internet publication].
https://www.healthquality.va.gov/guidelines/MH/sud
[51]Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018 Jan 1;175(1):86-90.
https://psychiatryonline.org/doi/book/10.1176/appi.books.9781615371969
http://www.ncbi.nlm.nih.gov/pubmed/29301420?tool=bestpractice.com
The use of pharmacological treatment does not preclude other psychosocial support; and psychosocial support does not preclude the use of pharmacological treatment. Indeed, the greatest efficacy may be seen in patients using a combination of drug therapy and psychosocial treatment.[77]Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA. 2018 Aug 28;320(8):815-24.
http://www.ncbi.nlm.nih.gov/pubmed/30167705?tool=bestpractice.com
However, patient choice to decline psychosocial treatment should not preclude or delay pharmacotherapy.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
Guideline recommendations on first-choice pharmacotherapy differ slightly. For patients with moderate-severe alcohol use disorder who desire abstinence, the American Psychiatric Association (APA) recommends offering naltrexone or acamprosate unless contraindicated, with choice guided by patient characteristics, mode of administration and potential adverse effects.[51]Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018 Jan 1;175(1):86-90.
https://psychiatryonline.org/doi/book/10.1176/appi.books.9781615371969
http://www.ncbi.nlm.nih.gov/pubmed/29301420?tool=bestpractice.com
Disulfiram is suggested as a second-choice option (for reasons of preference, intolerance or non-response) provided that the patient is aware of the risks from continued alcohol consumption. Topiramate or gabapentin (both off-label for this indication) are also suggested as second-line alternatives if indicated by ease of administration, preferred adverse effects profile or contraindications to other drugs.[51]Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018 Jan 1;175(1):86-90.
https://psychiatryonline.org/doi/book/10.1176/appi.books.9781615371969
http://www.ncbi.nlm.nih.gov/pubmed/29301420?tool=bestpractice.com
The VA/DoD recommends naltrexone or topiramate as first-choice options, with a weaker suggestion to offer acamprosate or disulfiram. Selection of topiramate is guided by demonstrated efficacy, including among veterans, specifically. Gabapentin is suggested if first-line drug therapy is contraindicated or ineffective.[42]US Department of Veterans Affairs. VA/DOD clinical practice guidelines: management of substance use disorders. 2021 [internet publication].
https://www.healthquality.va.gov/guidelines/MH/sud
Nonetheless, guideline groups are unanimous in emphasising the importance of informed patient choice, with patient preferences and care needs central to treatment planning.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
[51]Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018 Jan 1;175(1):86-90.
https://psychiatryonline.org/doi/book/10.1176/appi.books.9781615371969
http://www.ncbi.nlm.nih.gov/pubmed/29301420?tool=bestpractice.com
[42]US Department of Veterans Affairs. VA/DOD clinical practice guidelines: management of substance use disorders. 2021 [internet publication].
https://www.healthquality.va.gov/guidelines/MH/sud
It is suggested that drugs are continued for at least 6 months due to the chronic, relapsing nature of the condition.[77]Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA. 2018 Aug 28;320(8):815-24.
http://www.ncbi.nlm.nih.gov/pubmed/30167705?tool=bestpractice.com
Further treatment is determined through shared decision-making.
Naltrexone is an opioid receptor antagonist that decreases alcohol use by attenuating the rewarding and reinforcing effects of alcohol. Specifically, naltrexone blocks stimulation of the opioid receptor by endogenous opioids and decreases dopamine release in the ventral tegmental area.[91]Soyka M, Rösner S. Opioid antagonists for pharmacological treatment of alcohol dependence - a critical review. Curr Drug Abuse Rev. 2008 Nov;1(3):280-91.
http://www.ncbi.nlm.nih.gov/pubmed/19630726?tool=bestpractice.com
It is particularly useful in patients with a family history of alcohol use disorder and in those with significant alcohol cravings.[16]Swift RM. Drug therapy for alcohol dependence. N Engl J Med. 1999 May 13;340(19):1482-90.
http://www.ncbi.nlm.nih.gov/pubmed/10320388?tool=bestpractice.com
[51]Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018 Jan 1;175(1):86-90.
https://psychiatryonline.org/doi/book/10.1176/appi.books.9781615371969
http://www.ncbi.nlm.nih.gov/pubmed/29301420?tool=bestpractice.com
[92]Lingford-Hughes AR, Welch S, Peter L, et al. BAP updated guidelines: evidence-based guidelines for the pharmacological management of substance abuse, harmful use, addiction, and comorbidity: recommendations from BAP. J Psychopharmacol. 2012 Jul;26(7):899-952.
http://www.ncbi.nlm.nih.gov/pubmed/22628390?tool=bestpractice.com
Oral naltrexone can be started in patients who are still drinking, and it is usually well-tolerated. Naltrexone can precipitate opioid withdrawal in those with opioids in their system. It is not started until patients have been opioid-free for several days (7-10 days) and is contraindicated in those who require opioid agonists for pain or opioid use disorder. Naltrexone can cause stomach discomfort and a mild increase in liver function tests, especially in the oral formulation. As such, the oral formulation is not recommended in patients with alanine aminotransferase (ALT) measurements greater than 5 times the normal limit. Intramuscular naltrexone appears to be safe except in acute liver failure or decompensated cirrhosis. As it does not undergo first pass metabolism in the liver, serum concentration is more predictable and it is considered safer.[93]Antonelli M, Ferrulli A, Sestito L, et al. Alcohol addiction - the safety of available approved treatment options. Expert Opin Drug Saf. 2018 Feb;17(2):169-77.
http://www.ncbi.nlm.nih.gov/pubmed/29120249?tool=bestpractice.com
[94]Ciraulo DA, Dong Q, Silverman BL, et al. Early treatment response in alcohol dependence with extended-release naltrexone. J Clin Psychiatry. 2008 Feb;69(2):190-5.
http://www.ncbi.nlm.nih.gov/pubmed/18348601?tool=bestpractice.com
[95]Lucey MR, Silverman BL, Illeperuma A, et al. Hepatic safety of
once-monthly injectable extended-release naltrexone administered to actively
drinking alcoholics. Alcohol Clin Exp Res. 2008 Mar;32(3):498-504.
http://www.ncbi.nlm.nih.gov/pubmed/18241321?tool=bestpractice.com
Treatment with naltrexone reduces heavy drinking days, improves abstinence, and reduces the risk of returning to any or heavy drinking at 3 and 12 months post-treatment, compared with placebo.[96]Anton RF, O'Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006 May 3;295(17):2003-17.
http://jama.ama-assn.org/cgi/reprint/295/17/2003.pdf
http://www.ncbi.nlm.nih.gov/pubmed/16670409?tool=bestpractice.com
[  ]
What are the effects of opioid antagonists in people with alcohol dependence?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.601/fullShow me the answer In one meta-analysis of 118 RCTs, the number needed to treat (NNT) to prevent return to drinking for oral naltrexone was 18, and the NNT to reduce heavy drinking was 11.[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
The intramuscular formulation of naltrexone may be more effective in patients with difficulty taking daily drug treatments, with some evidence that it can reduce percentage drinking days (5% reduction vs. placebo).[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
[98]Pettinati HM, Silverman BL, Battisti JJ, et al. Efficacy of extended-release naltrexone in patients with relatively higher severity of alcohol dependence. Alcohol Clin Exp Res. 2011 Oct;35(10):1804-11.
http://www.ncbi.nlm.nih.gov/pubmed/21575016?tool=bestpractice.com
Naltrexone is supported as a first-line drug option for alcohol use disorder, in conjunction with psychosocial therapy.[51]Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018 Jan 1;175(1):86-90.
https://psychiatryonline.org/doi/book/10.1176/appi.books.9781615371969
http://www.ncbi.nlm.nih.gov/pubmed/29301420?tool=bestpractice.com
[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
[99]Bahji A, Bach P, Danilewitz M, et al. Pharmacotherapies for adults with alcohol use disorders: a systematic review and network meta-analysis. J Addict Med. 2022 Nov-Dec 01;16(6):630-8.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10010623
http://www.ncbi.nlm.nih.gov/pubmed/35653782?tool=bestpractice.com
It can be used as a monotherapy or in combination with gabapentin, topiramate, acamprosate, or disulfiram (unless specific drugs are contraindicated).
]
What are the effects of opioid antagonists in people with alcohol dependence?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.601/fullShow me the answer In one meta-analysis of 118 RCTs, the number needed to treat (NNT) to prevent return to drinking for oral naltrexone was 18, and the NNT to reduce heavy drinking was 11.[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
The intramuscular formulation of naltrexone may be more effective in patients with difficulty taking daily drug treatments, with some evidence that it can reduce percentage drinking days (5% reduction vs. placebo).[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
[98]Pettinati HM, Silverman BL, Battisti JJ, et al. Efficacy of extended-release naltrexone in patients with relatively higher severity of alcohol dependence. Alcohol Clin Exp Res. 2011 Oct;35(10):1804-11.
http://www.ncbi.nlm.nih.gov/pubmed/21575016?tool=bestpractice.com
Naltrexone is supported as a first-line drug option for alcohol use disorder, in conjunction with psychosocial therapy.[51]Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018 Jan 1;175(1):86-90.
https://psychiatryonline.org/doi/book/10.1176/appi.books.9781615371969
http://www.ncbi.nlm.nih.gov/pubmed/29301420?tool=bestpractice.com
[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
[99]Bahji A, Bach P, Danilewitz M, et al. Pharmacotherapies for adults with alcohol use disorders: a systematic review and network meta-analysis. J Addict Med. 2022 Nov-Dec 01;16(6):630-8.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10010623
http://www.ncbi.nlm.nih.gov/pubmed/35653782?tool=bestpractice.com
It can be used as a monotherapy or in combination with gabapentin, topiramate, acamprosate, or disulfiram (unless specific drugs are contraindicated).
Acamprosate normalises glutamate and gamma-aminobutyric acid neurotransmitter systems in the central nervous system. It is thought that these actions reduce ongoing symptoms associated with alcohol abstinence (e.g., anxiety, insomnia) and cravings. It is primarily metabolised by the kidneys, and a dose reduction may be required in patients with renal impairment (its use is contraindicated if creatinine clearance is <30 mL/minute).[51]Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018 Jan 1;175(1):86-90.
https://psychiatryonline.org/doi/book/10.1176/appi.books.9781615371969
http://www.ncbi.nlm.nih.gov/pubmed/29301420?tool=bestpractice.com
Acamprosate increases the rate and duration of abstinence, compared with placebo.[100]Rösner S, Hackl-Herrwerth A, Leucht S, et al. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD004332.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004332.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/20824837?tool=bestpractice.com
[101]Mason BJ, Lehert P. Acamprosate for alcohol dependence: a sex-specific meta-analysis based on individual patient data. Alcohol Clin Exp Res. 2012 Mar;36(3):497-508.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3288465
http://www.ncbi.nlm.nih.gov/pubmed/21895717?tool=bestpractice.com
Acamprosate is safe to use in patients who are actively drinking, but evidence for its use is from trials in people who have already stopped drinking. In meta-analysis, the NNT is 11 to prevent return to any drinking.[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
Diarrhoea is a common adverse effect.[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
Naltrexone and acamprosate are considered to be equally efficacious, with both supported as first-choice pharmacotherapy options.[51]Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018 Jan 1;175(1):86-90.
https://psychiatryonline.org/doi/book/10.1176/appi.books.9781615371969
http://www.ncbi.nlm.nih.gov/pubmed/29301420?tool=bestpractice.com
[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
[99]Bahji A, Bach P, Danilewitz M, et al. Pharmacotherapies for adults with alcohol use disorders: a systematic review and network meta-analysis. J Addict Med. 2022 Nov-Dec 01;16(6):630-8.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10010623
http://www.ncbi.nlm.nih.gov/pubmed/35653782?tool=bestpractice.com
[  ]
Can acamprosate (with or without naltrexone) support continued abstinence after detoxification in alcohol-dependent people?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.577/fullShow me the answer However, pill burden may reduce its effectiveness, and can limit use of acamprosate in clinical practice.
]
Can acamprosate (with or without naltrexone) support continued abstinence after detoxification in alcohol-dependent people?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.577/fullShow me the answer However, pill burden may reduce its effectiveness, and can limit use of acamprosate in clinical practice.
Gabapentin is approved for the treatment of partial seizures and neuropathic pain. The exact mechanism of gabapentin in alcohol use disorder is not known, but it appears to inhibit the release of excitatory neurotransmitters. In an RCT of 96 individuals with alcohol use disorder, gabapentin resulted in a statistically significant decrease in heavy drinking days (NNT 5) and prevented return to use (NNT 6) for patients with high baseline alcohol withdrawal symptoms.[102]Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020 May 1;180(5):728-36.
https://www.doi.org/10.1001/jamainternmed.2020.0249
http://www.ncbi.nlm.nih.gov/pubmed/32150232?tool=bestpractice.com
However, the largest RCT to date (346 patients), which used the extended-release formulation, found no benefit compared with placebo.[103]Falk DE, Ryan ML, Fertig JB, et al. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res. 2019 Jan;43(1):158-69.
https://pmc.ncbi.nlm.nih.gov/articles/PMC6317996
http://www.ncbi.nlm.nih.gov/pubmed/30403402?tool=bestpractice.com
Meta-analyses have concluded a low strength of evidence for gabapentin overall, with any effects on drinking outcomes being of only borderline statistical significance.[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
[99]Bahji A, Bach P, Danilewitz M, et al. Pharmacotherapies for adults with alcohol use disorders: a systematic review and network meta-analysis. J Addict Med. 2022 Nov-Dec 01;16(6):630-8.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10010623
http://www.ncbi.nlm.nih.gov/pubmed/35653782?tool=bestpractice.com
Nevertheless, gabapentin may be an effective option in patients with symptoms of alcohol withdrawal, or when other drugs are contraindicated or poorly tolerated.[42]US Department of Veterans Affairs. VA/DOD clinical practice guidelines: management of substance use disorders. 2021 [internet publication].
https://www.healthquality.va.gov/guidelines/MH/sud
[51]Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018 Jan 1;175(1):86-90.
https://psychiatryonline.org/doi/book/10.1176/appi.books.9781615371969
http://www.ncbi.nlm.nih.gov/pubmed/29301420?tool=bestpractice.com
It can be used as monotherapy or in conjunction with naltrexone.[104]Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011 Jul;168(7):709-17.
https://www.doi.org/10.1176/appi.ajp.2011.10101436
http://www.ncbi.nlm.nih.gov/pubmed/21454917?tool=bestpractice.com
The main side effects of gabapentin are dizziness, drowsiness, and nausea. Patients should be counselled that abrupt cessation of gabapentin can lower the seizure threshold.
Topiramate is an anticonvulsant that has been shown in RCTs to decrease craving and withdrawal symptoms and significantly improve physical and psychosocial well-being of patients with alcohol use disorder, compared with placebo.[105]Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003 May 17;361(9370):1677-85.
http://www.ncbi.nlm.nih.gov/pubmed/12767733?tool=bestpractice.com
[106]Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007 Oct 10;298(14):1641-51.
http://jama.ama-assn.org/cgi/content/full/298/14/1641
http://www.ncbi.nlm.nih.gov/pubmed/17925516?tool=bestpractice.com
[107]Johnson BA, Rosenthal N, Capece JA, et al. Improvement of physical health and quality of life of alcohol-dependent individuals with topiramate treatment: US multisite randomized controlled trial. Arch Intern Med. 2008 Jun 9;168(11):1188-99.
http://www.ncbi.nlm.nih.gov/pubmed/18541827?tool=bestpractice.com
[108]Shinn AK, Greenfield SF. Topiramate in the treatment of substance-related disorders: a critical review of the literature. J Clin Psychiatry. 2010 May;71(5):634-48.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3736141
http://www.ncbi.nlm.nih.gov/pubmed/20361908?tool=bestpractice.com
[109]Kenna GA, Lomastro TL, Schiesl A, et al. Review of topiramate: an antiepileptic for the treatment of alcohol dependence. Curr Drug Abuse Rev. 2009 May;2(2):135-42.
http://www.ncbi.nlm.nih.gov/pubmed/19630744?tool=bestpractice.com
In meta-analysis, compared with placebo, topiramate reduced percentage drinking days by 7%, heavy drinking days by 6%, and the number of drinks per drinking day by 2.[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
Importantly, efficacy has been established in subjects who were drinking at the time of starting the drug.[110]Swift RM. Topiramate for the treatment of alcohol dependence: initiating abstinence. Lancet. 2003 May 17;361(9370):1666-7.
http://www.ncbi.nlm.nih.gov/pubmed/12767727?tool=bestpractice.com
One study has shown that only individuals who are homozygous for the gene that codes for the kainate receptor protein had a reduction in heavy drinking days with topiramate treatment.[111]Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014 Apr;171(4):445-52.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3997125
http://www.ncbi.nlm.nih.gov/pubmed/24525690?tool=bestpractice.com
The benefits of topiramate should be weighed against the number needed to harm given its high side effect profile, including blurred vision, paraesthesias, poor memory, and loss of coordination.[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
Topiramate has been poorly tolerated in clinical trials, causing twice as many dropouts due to adverse effects as placebo.[99]Bahji A, Bach P, Danilewitz M, et al. Pharmacotherapies for adults with alcohol use disorders: a systematic review and network meta-analysis. J Addict Med. 2022 Nov-Dec 01;16(6):630-8.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10010623
http://www.ncbi.nlm.nih.gov/pubmed/35653782?tool=bestpractice.com
Disulfiram blocks the catabolic pathway of alcohol by inhibiting aldehyde dehydrogenase, thereby increasing acetaldehyde levels following alcohol ingestion. The disulfiram-alcohol reaction can produce a number of somatic effects: vasomotor symptoms (flushing), cardiovascular symptoms (tachycardia, hypotension), digestive symptoms (nausea, vomiting, diarrhoea), headache, respiratory depression, and malaise. These symptoms are generally transient, but serious reactions may occur that require urgent medical treatment. Due to hepatotoxicity, disulfiram is also contraindicated in patients with comorbid alcohol-related liver disease.[43]Jophlin LL, Singal AK, Bataller R, et al. ACG clinical guideline: alcohol-associated liver disease. Am J Gastroenterol. 2024 Jan 1;119(1):30-54.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11040545
http://www.ncbi.nlm.nih.gov/pubmed/38174913?tool=bestpractice.com
Disulfiram is prescribed for those intending to abstain from alcohol use, acting through negative reinforcement to promote abstinence. Trials to support disulfiram use are limited. One meta-analysis showed that disulfiram was more effective than placebo, acamprosate or naltrexone in open-label RCTs; a finding supported by subsequent network meta-analysis.[99]Bahji A, Bach P, Danilewitz M, et al. Pharmacotherapies for adults with alcohol use disorders: a systematic review and network meta-analysis. J Addict Med. 2022 Nov-Dec 01;16(6):630-8.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10010623
http://www.ncbi.nlm.nih.gov/pubmed/35653782?tool=bestpractice.com
[112]Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PLoS One. 2014;9(2):e87366.
https://www.doi.org/10.1371/journal.pone.0087366
http://www.ncbi.nlm.nih.gov/pubmed/24520330?tool=bestpractice.com
However, meta-analyses limited to blinded RCTs have not demonstrated a benefit of disulfiram.[97]McPheeters M, O'Connor EA, Riley S, et al. Pharmacotherapy for alcohol use disorder: a systematic review and meta-analysis. JAMA. 2023 Nov 7;330(17):1653-65.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10630900
http://www.ncbi.nlm.nih.gov/pubmed/37934220?tool=bestpractice.com
[112]Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PLoS One. 2014;9(2):e87366.
https://www.doi.org/10.1371/journal.pone.0087366
http://www.ncbi.nlm.nih.gov/pubmed/24520330?tool=bestpractice.com
Blinded studies are difficult to conduct, because the effectiveness of disulfiram depends on the patient's anticipation of somatic effects. Disulfiram therapy is more effective when supervised.[112]Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PLoS One. 2014;9(2):e87366.
https://www.doi.org/10.1371/journal.pone.0087366
http://www.ncbi.nlm.nih.gov/pubmed/24520330?tool=bestpractice.com
The following table summarises the drugs, their mechanism of action, and effectiveness for reducing drinking behaviour or to achieve abstinence.
[Figure caption and citation for the preceding image starts]: Summary of the drugs, their mechanism of action, and effectiveness for reducing drinking behaviour or to achieve abstinenceCreated by BMJ Knowledge Centre with information from: Anton RF et al. JAMA Intern Med 2020;180(5):728-36; McPheeters M et al. JAMA 2023;330(17):1653–1665; Falk DE et al. Alcohol Clin Exp Res 2019;43(1):158-169 [Citation ends].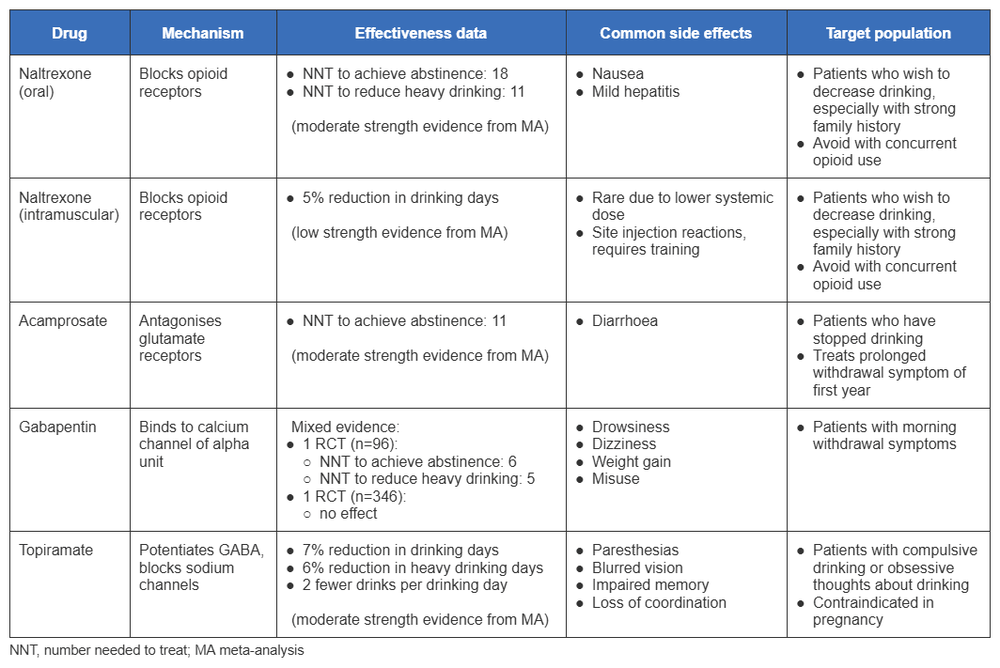
Evidence for the effectiveness of both psychosocial and pharmacological interventions for harmful alcohol use is from high-income countries, with no evidence available from low- and middle-income countries.[113]Greene MC, Kane J, Alto M, et al. Psychosocial and pharmacologic interventions to reduce harmful alcohol use in low- and middle-income countries. Cochrane Database Syst Rev. 2023 May 9;5(5):CD013350.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10167787
http://www.ncbi.nlm.nih.gov/pubmed/37158538?tool=bestpractice.com
Management of medical comorbidities
Patients with unhealthy alcohol use often have co-existing high blood pressure, insomnia, weight gain, cognitive disturbances, gastrointestinal distress, and cardiac arrhythmias. It is important to emphasise that many of these conditions may worsen with alcohol use, even at low levels. Management of physical comorbidities and timely integrated care is important to improve the well-being of the patient, and supports engagement in care.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
[114]AshaRani PV, Karuvetil MZ, Brian TYW, et al. Prevalence and correlates of physical comorbidities in alcohol use disorder (AUD): a pilot study in treatment-seeking population. Int J Ment Health Addict. 2022 Jan 23;1-18.
https://www.doi.org/10.1007/s11469-021-00734-5
http://www.ncbi.nlm.nih.gov/pubmed/35095353?tool=bestpractice.com
Chronic care management reduces harms of alcohol use disorder in patients with medical comorbidities.[115]Saitz R, Cheng DM, Winter M, et al. Chronic care management for dependence on alcohol and other drugs: the AHEAD randomized trial. JAMA. 2013 Sep 18;310(11):1156-67.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3902022
http://www.ncbi.nlm.nih.gov/pubmed/24045740?tool=bestpractice.com
Treatment programmes should have provision for external referral to medical services when indicated.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
Pregnant women
Psychosocial interventions may increase abstinence rates, compared with usual care or no intervention, in pregnant women who consume alcohol.[116]Ujhelyi Gomez K, Goodwin L, Jackson L, et al. Are psychosocial interventions effective in reducing alcohol consumption during pregnancy and motherhood? A systematic review and meta-analysis. Addiction. 2021 Jul;116(7):1638-63.
http://www.ncbi.nlm.nih.gov/pubmed/33067887?tool=bestpractice.com
[117]Minozzi S, Ambrosi L, Saulle R, et al. Psychosocial and medication interventions to stop or reduce alcohol consumption during pregnancy. Cochrane Database Syst Rev. 2024 Apr 29;4(4):CD015042.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11057221
http://www.ncbi.nlm.nih.gov/pubmed/38682758?tool=bestpractice.com
[  ]
How effective are psychological interventions for stopping or reducing alcohol consumption during pregnancy?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4453/fullShow me the answer It is unclear whether psychosocial therapies affect the number of drinks per day, though the evidence is overall of low quality, and predominantly relates to brief interventions.[117]Minozzi S, Ambrosi L, Saulle R, et al. Psychosocial and medication interventions to stop or reduce alcohol consumption during pregnancy. Cochrane Database Syst Rev. 2024 Apr 29;4(4):CD015042.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11057221
http://www.ncbi.nlm.nih.gov/pubmed/38682758?tool=bestpractice.com
[
]
How effective are psychological interventions for stopping or reducing alcohol consumption during pregnancy?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4453/fullShow me the answer It is unclear whether psychosocial therapies affect the number of drinks per day, though the evidence is overall of low quality, and predominantly relates to brief interventions.[117]Minozzi S, Ambrosi L, Saulle R, et al. Psychosocial and medication interventions to stop or reduce alcohol consumption during pregnancy. Cochrane Database Syst Rev. 2024 Apr 29;4(4):CD015042.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11057221
http://www.ncbi.nlm.nih.gov/pubmed/38682758?tool=bestpractice.com
[  ]
How effective are psychological interventions for stopping or reducing alcohol consumption during pregnancy?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4453/fullShow me the answer
]
How effective are psychological interventions for stopping or reducing alcohol consumption during pregnancy?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4453/fullShow me the answer
There are no RCTs evaluating the effectiveness of pharmacological interventions to reduce alcohol use or improve maternal, birth, and infant outcomes in pregnant women who consume alcohol or with alcohol use disorder.[117]Minozzi S, Ambrosi L, Saulle R, et al. Psychosocial and medication interventions to stop or reduce alcohol consumption during pregnancy. Cochrane Database Syst Rev. 2024 Apr 29;4(4):CD015042.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11057221
http://www.ncbi.nlm.nih.gov/pubmed/38682758?tool=bestpractice.com
[118]Smith EJ, Lui S, Terplan M. Pharmacologic interventions for pregnant women
enrolled in alcohol treatment. Cochrane Database Syst Rev. 2009 Jul 8;(3):CD007361.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD007361.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/19588428?tool=bestpractice.com
Due to the lack of evidence and increased risk of harm, the use of drug therapy during pregnancy is usually limited in clinical practice, where possible. However, given the impact of alcohol on pregnancy, pharmacotherapy may still be considered for women who are unable to stop drinking without pharmacological support.
Naltrexone is the best-studied in pregnant patients, although primarily in opioid use disorder, and is not associated with birth defects.[119]Towers CV, Katz E, Weitz B, et al. Use of naltrexone in treating opioid use disorder in pregnancy. Am J Obstet Gynecol. 2020 Jan;222(1):83.e1-83.e8.
http://www.ncbi.nlm.nih.gov/pubmed/31376396?tool=bestpractice.com
It is generally recommended to switch to the oral formulation at 36 weeks’ gestation to allow for effective pain management at birth.
Acamprosate is also an option if the benefits outweigh the risks, but its efficacy in this population is unclear.[120]DeVido J, Bogunovic O, Weiss RD. Alcohol use disorders in pregnancy. Harv Rev Psychiatry. 2015 Mar-Apr;23(2):112-21.
http://www.ncbi.nlm.nih.gov/pubmed/25747924?tool=bestpractice.com
Gabapentin may lead to withdrawal in neonates, especially in pregnant women with co-occurring alcohol use disorder.
Topiramate is contraindicated in pregnancy. Topiramate is associated with a higher risk of congenital malformations and a high prevalence of babies being born small for gestational age.[121]Medicines and Healthcare products Regulatory Agency. Topiramate (Topamax): introduction of new safety measures, including a pregnancy prevention programme. Jun 2024 [internet publication].
https://www.gov.uk/drug-safety-update/topiramate-topamax-introduction-of-new-safety-measures-including-a-pregnancy-prevention-programme
It may also be associated with an approximately 2 to 3 times increased risk of intellectual disability, autistic spectrum disorders and attention deficit hyperactivity disorder.[122]Bjørk MH, Zoega H, Leinonen MK, et al. Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. 2022 Jul 1;79(7):672-81.
https://jamanetwork.com/journals/jamaneurology/fullarticle/2793003
http://www.ncbi.nlm.nih.gov/pubmed/35639399?tool=bestpractice.com
In some countries, topiramate is contraindicated in pregnancy and in women of child-bearing age unless the conditions of a pregnancy prevention programme are fulfilled to ensure that women of child-bearing potential: are using highly effective contraception; have a pregnancy test to exclude pregnancy before starting topiramate; and are aware of the risks associated with use of the drug.[121]Medicines and Healthcare products Regulatory Agency. Topiramate (Topamax): introduction of new safety measures, including a pregnancy prevention programme. Jun 2024 [internet publication].
https://www.gov.uk/drug-safety-update/topiramate-topamax-introduction-of-new-safety-measures-including-a-pregnancy-prevention-programme
[123]European Medicines Agency. PRAC recommends new measures to avoid topiramate exposure in pregnancy. Sep 2023 [internet publication].
https://www.ema.europa.eu/en/news/prac-recommends-new-measures-avoid-topiramate-exposure-pregnancy
Disulfiram is not recommended in pregnant patients due to its gastrointestinal side effects and lack of safety data.
Adolescents
One meta-analysis of 16 studies demonstrated that psychosocial interventions are effective in reducing adolescent alcohol use, with individual-directed treatments possibly having a greater effect than family-based ones. Interventions with large effect sizes included brief motivational interviewing, cognitive behavioural therapy with 12 steps, cognitive behavioural therapy with aftercare, multidimensional family therapy, brief interventions with the adolescent, and brief interventions with the adolescent and a parent.[124]Tripodi SJ, Bender K, Litschge C, et al. Interventions for reducing adolescent alcohol abuse: a meta-analytic review. Arch Pediatr Adolesc Med. 2010 Jan;164(1):85-91.
http://archpedi.ama-assn.org/cgi/content/full/164/1/85
http://www.ncbi.nlm.nih.gov/pubmed/20048247?tool=bestpractice.com
Group therapy-based treatments may be effective for adolescents, but safety should be considered and these modalities require further investigation.[125]Engle B, Macgowan MJ. A critical review of adolescent substance abuse group treatments. J Evid Based Soc Work. 2009 Jul;6(3):217-43.
http://www.ncbi.nlm.nih.gov/pubmed/20183675?tool=bestpractice.com
Naltrexone has been found to reduce cravings in adolescents in a few small trials. Acamprosate has likewise shown promise. Disulfiram can be useful for adolescents motivated to stop drinking, but has poor efficacy in those who do not desire abstinence and are not receiving it in directly observed therapy.[126]Clark DB. Pharmacotherapy for adolescent alcohol use disorder. CNS Drugs. 2012 Jul 1;26(7):559-69.
http://www.ncbi.nlm.nih.gov/pubmed/22676261?tool=bestpractice.com
However, given the overall lack of evidence and increased risk of harm, the use of drug therapy in adolescents is usually limited in clinical practice, where possible.
Concurrent opioid use
It is recommended that patients have access to evidence-based treatment for all substance use concerns, and that secondary substance use is not considered a barrier to care.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
Several step-by-step treatment recommendations have been developed to integrate psychosocial therapies with pharmacological therapies for alcohol and drug use disorders.[127]O'Malley SS, Carroll KM. Psychotherapeutic considerations in pharmacological trials: alcoholism, clinical and experimental research. Alcohol Clin Exp Res. 1996 Oct;20(7 suppl):17A-22A.
http://www.ncbi.nlm.nih.gov/pubmed/8904990?tool=bestpractice.com
[128]Pettinati HM, Volpicelli JR, Pierce JD, et al. Improving naltrexone response: an intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. J Addict Dis. 2000;19(1):71-83.
http://www.ncbi.nlm.nih.gov/pubmed/10772604?tool=bestpractice.com
These procedures include patient education, personalised feedback, emotional support, drug monitoring, and motivational enhancement.
National Institute on Alcohol Abuse and Alcoholism (NIAAA)
Opens in new window
Choice of first-line pharmacological treatment options vary in people with concurrent opioid use. Naltrexone can precipitate opioid withdrawal in those with opioids in their system, so should be avoided. It is not started in patients until they have been opioid-free for several days (7-10 days) and is contraindicated in patients who require opioid agonists for pain or opioid use disorder.
Acamprosate and gabapentin may be used in patients with concurrent opioid use and alcohol use disorder. Evidence indicates that patients with opioid use disorder and polydrug users are at risk for gabapentin misuse and clinicians should monitor usage closely.[129]Modesto-Lowe V, Barron GC, Aronow B, et al. Gabapentin for alcohol use disorder: a good option, or cause for concern? Cleve Clin J Med. 2019 Dec;86(12):815-23.
https://www.doi.org/10.3949/ccjm.86a.18128
http://www.ncbi.nlm.nih.gov/pubmed/31821139?tool=bestpractice.com
Patients should also be counselled that abrupt cessation can lower seizure threshold.
Topiramate and disulfiram are second-line options.
Sedating substances or drug (e.g., benzodiazepines, barbiturates, opioids, muscle relaxants) may have similar signs and symptoms as alcohol use disorder, or may be used together with alcohol. Use of these substances with alcohol may pose risk for overdose complications (e.g., respiratory depression).
Concurrent mental health diagnosis
Symptoms of alcohol use disorder (including those of intoxication/withdrawal) may be confused with symptoms of other psychiatric disorders. Furthermore, other psychiatric disorders can co-occur with alcohol use disorder. Observation over days to weeks to months may be necessary to clarify underlying psychiatric diagnoses. However, co-treatment generally has better outcomes than waiting for patients to stop drinking before making a diagnosis or starting psychiatric treatment; therefore, treatment should not be withheld until patients stop drinking.
Psychosocial treatments for alcohol use disorder, and naltrexone, can be used first-line. However, note that evidence (from high-income countries) on the effectiveness of psychosocial interventions in alcohol use disorder primarily relates to patient populations without comorbid mental health illness, with no studies including people with severe mental health illness.[87]World Health Organization. Mental Health Gap Action Programme (mhGAP) guideline for mental, neurological and substance use disorders, third edition. Nov 2023 [internet publication].
https://www.who.int/publications/i/item/9789240084278
Acamprosate, gabapentin, topiramate, or disulfiram may be considered as alternative drug options to naltrexone, with selection guided by contraindications, tolerance, and patient preference.
One meta-analysis of 15 RCTs demonstrated reduced alcohol use and improved psychiatric outcomes when co-occurring depressive and anxiety disorders were treated concurrently with alcohol use disorder.[130]Hobbs JD, Kushner MG, Lee SS, et al. Meta-analysis of supplemental treatment for depressive and anxiety disorders in patients being treated for alcohol dependence. Am J Addict. 2011 Jul-Aug;20(4):319-29.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3124006
http://www.ncbi.nlm.nih.gov/pubmed/21679263?tool=bestpractice.com
Treatment options depend on diagnosis and may include antidepressants. Selective serotonin-reuptake inhibitors (SSRIs) have demonstrated efficacy in alcohol use disorder for patients with comorbid depression. Sertraline, when compared with placebo, decreases the proportion of days drinking during treatment and increases the number of patients who had continuous abstinence. Its efficacy was higher in those with more mild alcohol use disorder.[131]Pettinati HM, Volpicelli JR, Kranzler HR, et al. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000 Jul;24(7):1041-9.
http://www.ncbi.nlm.nih.gov/pubmed/10924008?tool=bestpractice.com
Trials of fluoxetine for alcohol use disorder achieved similar results, suggesting that fluoxetine may also be effective in mild alcohol use disorder.[132]Kranzler HR, Burleson JA, Brown J, et al. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996 Dec;20(9):1534-41.
http://www.ncbi.nlm.nih.gov/pubmed/8986200?tool=bestpractice.com
However, not all studies have supported the utility of SSRIs for treating alcohol use disorder. One study of fluoxetine for people with alcohol use disorder and co-occurring depression demonstrated no benefit of fluoxetine versus placebo on either depression or drinking.[133]Cornelius JR, Bukstein OG, Wood DS, et al. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009 Oct;34(10):905-9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2720419
http://www.ncbi.nlm.nih.gov/pubmed/19321268?tool=bestpractice.com
For more details on management of depression see Depression in adults.
Treatment programmes should have provision for external referral to psychiatric services, or to a more intensive level of care, when indicated.[3]American Society of Addiction Medicine. Engagement and retention of nonabstinent patients in substance use treatment: clinical consideration for addiction treatment providers. Oct 2024 [internet publication].
https://www.asam.org/quality-care/clinical-recommendations/asam-clinical-considerations-for-engagement-and-retention-of-non-abstinent-patients-in-treatment
In patients with suspected or diagnosed bipolar disorder, psychiatric consultation is strongly recommended.

 ]
Brief interventions may be improved by follow-up; for instance, one multi-site randomised controlled trial (RCT) in the trauma setting showed improvement in several drinking measures at up to 12 months for patients who received an individualised follow-up phone call in addition to brief motivational interviewing, compared with those who just received the interview.[82] A formal means of assessing the effectiveness of the plan and follow-up visits with the clinician should be part of the plan.[83] Though brief interventions are easy to administer, they go beyond simply telling patients to reduce their alcohol use: in studies of their effectiveness, clinicians were trained to provide structured feedback based on an individual’s risk and desire to change their behaviour. One commonly used structure is the FRAMES method: Feedback, Responsibility, Advice, Menu of options, Empathic style, Self-efficacy.
]
Brief interventions may be improved by follow-up; for instance, one multi-site randomised controlled trial (RCT) in the trauma setting showed improvement in several drinking measures at up to 12 months for patients who received an individualised follow-up phone call in addition to brief motivational interviewing, compared with those who just received the interview.[82] A formal means of assessing the effectiveness of the plan and follow-up visits with the clinician should be part of the plan.[83] Though brief interventions are easy to administer, they go beyond simply telling patients to reduce their alcohol use: in studies of their effectiveness, clinicians were trained to provide structured feedback based on an individual’s risk and desire to change their behaviour. One commonly used structure is the FRAMES method: Feedback, Responsibility, Advice, Menu of options, Empathic style, Self-efficacy. 
 ]
[Evidence A] Self-help programmes such as AA can provide valuable additional support for many patients and their families. Patients indicating benefit from support group attendance should be encouraged to attend the group meetings over an extended timeframe; some patients may benefit from attending indefinitely. In one large RCT, AA improved drinking outcomes, largely through improvements in adaptive social network changes and social self-efficacy.[88] Each AA group is independent, so patients should be encouraged to try several if one is not a good fit. For those who have negative experiences or views of AA, it is important that clinicians are aware of alternatives, such as Smart Recovery groups.
]
[Evidence A] Self-help programmes such as AA can provide valuable additional support for many patients and their families. Patients indicating benefit from support group attendance should be encouraged to attend the group meetings over an extended timeframe; some patients may benefit from attending indefinitely. In one large RCT, AA improved drinking outcomes, largely through improvements in adaptive social network changes and social self-efficacy.[88] Each AA group is independent, so patients should be encouraged to try several if one is not a good fit. For those who have negative experiences or views of AA, it is important that clinicians are aware of alternatives, such as Smart Recovery groups. 
 ]
In one meta-analysis of 118 RCTs, the number needed to treat (NNT) to prevent return to drinking for oral naltrexone was 18, and the NNT to reduce heavy drinking was 11.[97] The intramuscular formulation of naltrexone may be more effective in patients with difficulty taking daily drug treatments, with some evidence that it can reduce percentage drinking days (5% reduction vs. placebo).[97][98] Naltrexone is supported as a first-line drug option for alcohol use disorder, in conjunction with psychosocial therapy.[51][97][99] It can be used as a monotherapy or in combination with gabapentin, topiramate, acamprosate, or disulfiram (unless specific drugs are contraindicated).
]
In one meta-analysis of 118 RCTs, the number needed to treat (NNT) to prevent return to drinking for oral naltrexone was 18, and the NNT to reduce heavy drinking was 11.[97] The intramuscular formulation of naltrexone may be more effective in patients with difficulty taking daily drug treatments, with some evidence that it can reduce percentage drinking days (5% reduction vs. placebo).[97][98] Naltrexone is supported as a first-line drug option for alcohol use disorder, in conjunction with psychosocial therapy.[51][97][99] It can be used as a monotherapy or in combination with gabapentin, topiramate, acamprosate, or disulfiram (unless specific drugs are contraindicated). ]
However, pill burden may reduce its effectiveness, and can limit use of acamprosate in clinical practice.
]
However, pill burden may reduce its effectiveness, and can limit use of acamprosate in clinical practice.
 ]
It is unclear whether psychosocial therapies affect the number of drinks per day, though the evidence is overall of low quality, and predominantly relates to brief interventions.[117]
[
]
It is unclear whether psychosocial therapies affect the number of drinks per day, though the evidence is overall of low quality, and predominantly relates to brief interventions.[117]
[  ]
]