FDA suspends biologics license of live-attenuated chikungunya vaccine (Ixchiq®) in the US
The US Food and Drug Administration (FDA) has suspended the biologics license of the live-attenuated chikungunya vaccine (known commercially as Ixchiq®) in the US. The suspension is effective immediately (as of 22 August 2025) and requires the manufacturer to stop shipping and selling the vaccine in the US. The FDA believes that the vaccine is not safe as it appears to be causing chikungunya-like illness in recipients, and continued administration would pose a danger to public health.[49]
The suspension follows the FDA’s decision on 6 August 2025 to remove a pause in the use of the vaccine in people aged ≥60 years. At the time, the agency approved updates to the prescribing information including revising the indication, adding limitations of use, and adding enhanced warnings and precautions to reflect serious adverse events picked up during postmarketing surveillance.[50] However, since then, four new cases of serious adverse events have been reported in the Vaccine Adverse Event Reporting System (VAERS), prompting the FDA to suspend the vaccine’s license.[51]
In May 2025, use of the live-attenuated vaccine was paused in older patients (aged ≥60 years in the US and ages ≥65 years in Europe) due to post-marketing reports of serious adverse events, including cardiac and neurological events, in people who received the vaccine.[52][53] At the time, a review of the safety data revealed 28 cases of serious adverse effects, including encephalitis and chikungunya-like symptoms, and three deaths. The serious adverse effects were mainly reported in people aged ≥65 years and those with multiple or uncontrolled medical conditions. The adverse effects led to a worsening of the patients’ medical conditions or a deterioration in their general health, sometimes leading to hospitalisation and death. Cases of encephalitis were rare.[54]
The live-attenuated vaccine continues to be available in countries where it is still licensed.[51]
In Europe, the European Medicines Agency (EMA) also paused use of the vaccine in people ages ≥65 years in May 2025, but lifted the restriction in July 2025. The EMA currently recommends that, in people of all ages, the live-attenuated vaccine should only be given when there is a significant risk of chikungunya virus infection, and only after careful consideration of the risks and benefits of vaccination.[54]
In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) has temporarily restricted use of the vaccine in people ages ≥65 years while it reviews the data.[55]
The live-attenuated vaccine was initially approved by the FDA under the accelerated approval pathway in November 2023. The clinical benefit of the vaccine has not yet been confirmed in clinical studies. It was the first vaccine to be approved for the prevention of disease caused by chikungunya virus. Over 80,000 doses have been used worldwide since its approval.
An adjuvanted recombinant virus-like particle vaccine (known commercially as Vimkunya®) was recently approved in the US and Europe for the prevention of disease caused by chikungunya virus for people aged ≥12 years. There have been no safety issues reported with this vaccine as yet.
Summary
Definition
History and exam
Key diagnostic factors
- presence of risk factors
- history of mosquito bites
- fever
- arthralgias/arthritis
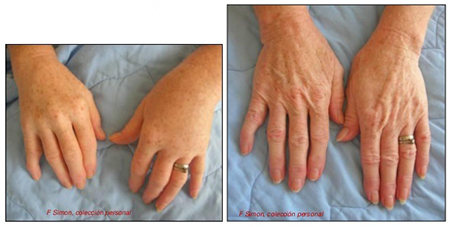
- rash and other dermatological manifestations
Other diagnostic factors
- comorbid illness
- back pain
- neuropathic-type pain
- headache
- lymphadenopathy
- ocular manifestations
- photophobia
- sore throat
- gastrointestinal manifestations
- neurological manifestations
- haemorrhagic manifestations
Risk factors
- Aedes mosquito bites
- travel to/residence in endemic area
- outdoor exposure
- environmental factors favouring breeding of mosquitoes
- neonate with infected mother
- low educational level
- age >40 years
- male sex
- blood transfusion
- blood group O positive
- comorbid illnesses (risk of more severe disease)
- neonate (risk of more severe disease)
Diagnostic investigations
1st investigations to order
- FBC with differential
- LFTs
- erythrocyte sedimentation rate
- CRP
- basic metabolic panel
- serology
- molecular testing
Investigations to consider
- musculoskeletal imaging
- MRI brain
- cerebrospinal fluid analysis
- electromyogram
- nerve conduction studies
- electroencephalogram
- placental histology
Treatment algorithm
Contributors
Authors
Miguel G. Madariaga, MD, MSc, FACP

Infectious Diseases Consultant
Naples Community Hospital
Naples
FL
Disclosures
MGM declares that he has no competing interests.
Peer reviewers
Jessica Fairley, MD
Assistant Professor of Medicine
Division of Infectious Diseases
Emory University
Atlanta
GA
Disclosures
JF declares that she has no competing interests.
Mala Chhabra, MBBS, MD
Joint Director
National Centre for Disease Control
Delhi
India
Disclosures
MC declares that she has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
Centers for Disease Control and Prevention. Clinical testing and diagnosis for chikungunya virus disease. May 2024 [internet publication].Full text
Brito CAA, Marques CDL, Falcão MB, et al. Update on the treatment of musculoskeletal manifestations in chikungunya fever: a guideline. Rev Soc Bras Med Trop. 2020;53:e20190517.Full text Abstract
World Health Organization. WHO guidelines for clinical management of arboviral diseases: dengue, chikungunya, Zika and yellow fever. Jul 2025 [internet publication].Full text
World Health Organization. Guidelines on clinical management of Chikungunya fever. Dec 2019 [internet publication].Full text
Centers for Disease Control and Prevention. Treatment and prevention of chikungunya virus disease. May 2025 [internet publication].Full text
Reference articles
A full list of sources referenced in this topic is available here.
Use of this content is subject to our disclaimer